1. Introduction
Recent translational studies indicate that glucocorticoid receptor (GR) activation increases tumour heterogeneity and metastasis formation, suggesting caution in using glucocorticoids along with chemotherapy for breast cancer patients [
1,
2,
3,
4]. In preclinical studies, increased glucocorticoid receptor activity was demonstrated in triple-negative, metastatic breast cancer that led to increased colonisation on metastatic sites and reduced survival [
2]. Chemotherapy (based on anthracyclines and taxanes) is the standard treatment strategy for metastatic triple-negative breast cancer (TNBC) [
5]. Glucocorticoids are routinely administered as supportive therapy to counteract adverse symptoms associated with chemotherapy and radiation therapy [
6,
7]. Dexamethasone ameliorates chemotherapy-induced cytotoxicity and prevents allergic reactions [
6,
7]. Additionally, glucocorticoids inhibit anti-tumour immune responses and are linked to increased tumour cell survival [
7] that could potentially influence the effect of immunotherapy as well.
First, on the RNA level, Pan et al. demonstrated that high GR expression was associated with worse survival in TNBC [
8]. However, immunohistochemistry studies have been somewhat controversial and no subtype-specific data have been reported despite the observation of RNA-based studies that in oestrogen receptor-positive (ER+) breast cancer, high GR gene expression correlated with good outcome [
9,
10,
11,
12,
13,
14,
15].
GR, a nuclear receptor, is located in the cytoplasm in its unliganded form. Upon ligand binding, GR undergoes a conformational change, translocates to the nucleus and modifies gene expression [
16,
17]. Additionally, GR has nongenomic effects in the cytoplasm, such as activating the phosphoinositide 3-kinase/protein kinase B pathway and NF-κB, regulating JNK function, inducing apoptosis, and affecting metabolic pathways through mitochondrial and membrane-bound GR [
16,
17].
Immunohistochemical (IHC) studies of normal breast tissue show GR positivity in both cytoplasm and nuclei [
18,
19]. Furthermore, Conde et al. have reported that during breast cancer development, GR shifts from the nucleus to the cytoplasm [
20].
Several GR isoforms, including GRα and GRβ, diversify GR function. GRβ, an inhibitor of GRα, is produced through alternative splicing, affecting its ligand-binding domain [
17,
21]. It is described that GRβ is expressed in tissues at lower levels compared to GRα or not expressed until a disease state, such as cancer, or prolonged glucocorticoid treatment occurs [
21]. It is suggested that the subcellular localisation of GRβ depends on cell type [
21,
22]. In breast tissue, we previously demonstrated the presence of GRβ in both normal and cancerous breast specimens mostly in cytoplasmic localisation in tumours, while some samples also showed nuclear positivity [
19]. Within the cell nucleus, GRβ competes with GRα for binding to glucocorticoid response elements (GREs), forms heterodimers with GRα, interacts with proteins that prevent GRα activity, recruits deacetylase complexes, and has GRα-independent transcriptional activity [
21]. However, the cytoplasmic and nuclear function of GRβ in breast cancer remain controversial [
21].
Previous IHC reports have used antibodies that detect only or predominantly nuclear GR, regardless of isoform composition [
9,
10,
11,
12,
13,
14,
15,
23,
24]. Therefore, we are lacking data on GRβ’s clinicopathological relevance due to difficulties in discriminating GRβ from the other GR isoforms.
Therefore, in this study, using a specific and validated antibody [
19] against GRβ, we aimed to analyse the intracellular localisation of the total GR (GRtotal) and the β isoform of the GR (GRβ) in vitro using transfection of plasmid constructs encoding the GR isoforms and confocal fluorescent immunocytochemistry. As a second objective, we performed immunostaining for GRtotal and GRβ on two clinical breast cancer cohorts: (1) one cohort that consisted of 194 clinical breast cancer samples to compare different molecular subtypes, and (2) a cohort of 161 TNBC samples to evaluate the association of GRtotal and GRβ on survival.
3. Discussion
Previously, only nuclear GR staining was evaluated in relation to clinicopathological parameters in breast cancer [
9,
10,
11,
12,
13,
14,
15,
23,
24]. However, the presence of cytoplasmic GR has important non-transcriptomic functions in the breast, and a shift from nuclear to cytoplasmic GR has been noted during breast cancer development [
17,
18,
19,
20,
25]. Consistent with this, we detected GRt-cpl and GRβ-cpl in 85% and 80% of all breast cancer samples, respectively, indicating a high prevalence and potential significance. Using a different antibody, Al-Alem observed cytoplasmic GR in 43% of samples [
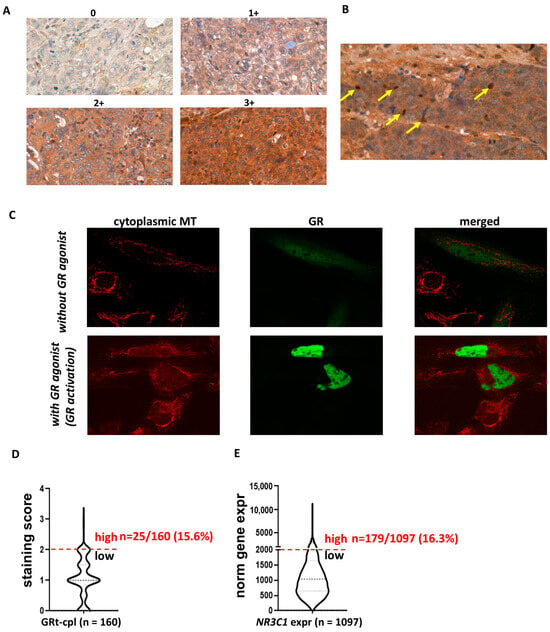
10]. Among the human breast cancer specimens, we found overall weak-to-moderate cytoplasmic GRtotal (GRt-cpl) and GRβ (GRβ-cpl) staining. We verified the small proportion of high GRt-cpl samples (16.3%) with an independent set of over 1000 breast cancer samples and found a similar rate.
The literature reports on nuclear GR expression are heterogeneous, ranging from a consistent lack of GR staining in almost all invasive breast carcinomas (except metaplastic tumours) to varying positivity rates between 44% and 83% [
9,
10,
12,
24]. In our study, nuclear GR positivity was less frequent, with 26% and 7% for GRtotal and GRβ isoform, respectively. Additionally, GR staining parameters were not independent of each other; positive correlations were observed between GRt-cpl and GRβ-cpl, and between GRt-cpl and GRt-nucl [
26]. The correlation of GR presence with clinicopathological parameters in the literature is also highly controversial. Some authors found no correlation between GR and breast cancer subtypes or characteristics [
10]. However, in line with other reports, we found a fairly consistent positive correlation between GRt-cpl and ER [
9]. GR expression was higher in ER+ breast cancer compared to ER- tumours and showed low expression in TNBC [
9,
23]. Similarly to our results, higher GR staining has been observed in lobular compared to ductal breast tumours [
10].
Based on literature data, the function of GR depends on the presence of ER [
27], so we investigated the correlation of GR staining in LumA and TNBC samples. In ER+ samples, in accordance with others [
9], we found that GRt-cpl negatively correlated with Ki67, supported by the comparisons of LumA and LumB-Her2- samples. In TNBC, however, GRt-cpl and GRβ-cpl showed an opposite trend. Indeed, it has been shown that activated GR binds to and represses the enhancer regions of the ER-mediated cell cycle genes’ (e.g.,
CCND1,
CDK2, and
CDK6) in ER+ breast cancer cells [
28]. The interaction between ER and GR was reported to have a clinical impact, as in ER+ breast cancer low GR expression associated with worse outcomes and high Ki67 [
8,
9]. TNBC samples with strong lymphoid infiltration had higher GRt-cpl, while those with extensive vascular infiltration had higher GRβ-cpl. This phenomenon requires further research, as GR generally has an immune-suppressive nature and GRβ has been linked to glucocorticoid resistance [
29]. These findings could impact tumour-immune surveillance and influence immune-checkpoint inhibitor treatment efficacy in TNBC patients [
30,
31], but these hypothesis needs further studies.
In our univariate analysis, high GRt-nucl staining associated with worse progression-free survival, but this significance was lost in multivariate analysis. This aligns with Abduljabbar et al., who found a similar pattern for breast-cancer-specific survival [
9].
Clarifying the role of GRβ in breast cancer is a high priority due to its suggested antagonistic effect compared to the main GR isoform GRα. However, there is limited information on GR splice variants in clinical breast cancer specimens [
21,
32,
33,
34,
35,
36,
37]. To our knowledge, no immunohistochemistry studies on the GRβ isoform in breast cancer have been published. The sequence similarity between GRα and GRβ makes specific discrimination challenging [
19]. Therefore, we developed and validated an antibody against GRβ [
19]. Using this antibody, we observed an opposite trend in association of GRβ-cpl with survival compared to cytoplasmic GRtotal. In multivariate analysis, samples with high GRβ-cpl staining showed significantly longer OS compared to low GRβ-cpl samples. This may be due to the different transcriptional and cytoplasmic actions of the GRβ isoform [
21]. While GRα and GRβ can influence each other’s activity, GRβ also has intrinsic, GRα-independent transcriptional activities [
38,
39]. Indeed, in breast cancer, we observed different effects of GRα and GRβ on proliferation, migration, and apoptosis in the context of ER [
19].
Several studies have reported GR’s non-genomic, cytoplasmic effects through protein–protein interactions and post-translational modifications [
40]. In our cohorts, GRβ showed lower nuclear presence compared to GRtotal. Though, the cytoplasmic function of GRβ in breast cancer cells remains unknown.
Although cytoplasmic staining is challenging to assess in clinical samples, the amount of GRβ-cpl may serve as a useful marker to identify breast cancer patients with different outcomes.
5. Conclusions
GRtotal and GRβ expression may have important relevance for patients with TNBC who receive supporting glucocorticoids (most frequently dexamethasone) as premedication to prevent hypersensitivity reactions to chemotherapy and as antiemetics.
Previous studies demonstrated that GR activation promotes disease progression in both cell line and patient-derived xenograft models [
2]. It was also shown that even in the absence of the ligand, GR activation increases breast cancer cell migration in TNBC [
3,
4]. In addition, the GRβ isoform, which has an effect opposite to the main isoform GRα, plays an important role in breast cancer cell behaviour [
19].
Based on our previous and published data, we aimed to take a step forward and validate the association of GR with clinical outcomes at the protein level using immunohistochemistry, a method routinely applied in pathology to characterise molecular subtypes through ER, PR, Her2, and Ki67 staining. Our data showed that GR can be detected in the cytoplasm of breast cancer cells and isoform-specific antibodies are crucial for detecting GR in breast cancer and understanding its clinicopathological associations, which may explain the conflicting literature findings.
We demonstrated an opposite association between GR and the proliferation index in the context of oestrogen receptor status. Based on this finding, survival analysis was performed on TNBC, where nuclear GRtotal and cytoplasmic GRβ showed opposing associations with patient outcomes. GRtotal was associated with a worse prognosis, while cytoplasmic localisation of GRβ correlated with a better prognosis in the multivariate analysis. These findings suggest that cellular localisation and GR isoforms may function differently in breast cancer, highlighting the multidirectional potential of GR. Our study is the first to evaluate the association of the GRβ isoform with clinicopathological parameters in breast cancer progression. Therefore, further investigations are needed before GR staining can be implemented into routine clinical practice.