1. Introduction
The olive (Olea europaea) is an important economic and widespread woody oil plant native to the Mediterranean region [1,2]. Olive oil is rich in nutrients such as unsaturated fatty acids (MUFA), phenolic compounds, and vitamin E; it is widely popular. In particular, the oleocanthal is beneficial to human health, as it may be helpful for treating obesity, cardiovascular disease, diabetes, hypertension, and other diseases [3,4]. In addition, the secondary metabolites in olive leaves are second only to olive oil in importance. Olive leaves contain a large number of antioxidants, such as oleuropein, hydroxytyrosol, tyrosol, as well as caffeic acid and lignans [5]. These natural substances have antioxidant, antibacterial, antiviral, and anti-inflammatory properties, and are thus of great value in the field of medicine [6,7].
As the nutritional benefits of olives have become more widely recognized, olive cultivation has gradually expanded from Mediterranean countries, such as Spain [8], Italy [9], and Turkey [10], to non-Mediterranean regions, including China [11] and Argentina [12]. Introduced to China in 1956, olives are now predominantly cultivated in provinces like Yunnan, Gansu, Sichuan, and Shaanxi [13,14]. With the expansion of both the planting area and regions, olive cultivation is encountering an increasing number of climate-related challenges. In the face of global climate change, it is particularly crucial to select and breed olive germplasm resources that can thrive in a variety of climatic conditions. Additionally, understanding how olive’s adaptation to environments, and identifying its key genes are prerequisites for breeding new stress-resistant germplasm [15].
Transcription factors (TFs) play pivotal roles in the signal transduction pathways that plants use to respond to abiotic stress. Among these TFs, the Apetala2/Ethylene Responsive Factor (AP2/ERF) superfamily stands out as one of the largest and most influential families. Members of this superfamily are integral to the regulation of plant growth and development, as well as to the responses to both biotic and abiotic stressors [16,17]. The C-repeat Binding Factor/Dehydration Responsive Element Binding (CBF/DREB1) primarily regulates the expression of numerous genes that respond to stress independent on abscisic acid (ABA) signal pathway. This regulation is crucial for protecting plants against the detrimental effects of frost and drought, which are characterized by water loss. CBF/DREB1 achieves this by binding to the C-repeat/dehydration response element (CRT/DRE) found in the promoter regions of various genes, often referred as ‘CBF regulators’. In response to abiotic stress, these genes contribute to osmotic protection through the synthesis of protective proteins, modulation of carbohydrate metabolism, and regulation of sugar transport [18,19,20]. Today, CBF/DREB1 genes have been identified across a range of plant species, such as Arabidopsis [21,22], barley [15,20], rice [15,21], tobacco [23], tea [24], cassava [25], potato [19], and lettuce [26]. Moreover, the ICE-CBF-COR cascade was identified as a vital cold response pathway in plants that mitigate the effects of cold stress [27,28]. It consists of three main components: the CBF, the CBF expression inducer (ICE), and the cold response (COR) genes [15,28,29]. Additionally, the overexpression of the Arabidopsis CBF3 gene could increase cassava’s tolerance to cold and drought stress [25]. The overexpression of tea CBF3 in Arabidopsis resulted in enhanced cold tolerance [23]. Obviously, identifying and understanding these CBF pathway genes are essential for improving freezing tolerance in agricultural crops.
The olive genome was first reported in 2016 [30]; however, the shotgun sequencing approach used at the time resulted in an incomplete assembly. Advances in genomics led to a more refined olive genome in 2021, when Rao [31] employed the Oxford Nanopore third-generation sequencing coupled with Hi-C technology. This facilitated the identification of nine gene families, encompassing 202 genes involved in oleuropein biosynthesis, as well as 14 gene families that included 128 genes linked to the fatty acid biosynthesis pathway. Focusing on the genetic basis of olive cold stress, our study specifically targeted genes within the ERF family, particularly the CBF genes. We conducted a thorough analysis to elucidate their roles and the underlying regulatory mechanisms in cold stress response. The insights gained from this research are instrumental for the strategic selection and breeding of olive varieties with enhanced cold tolerance.
4. Discussion
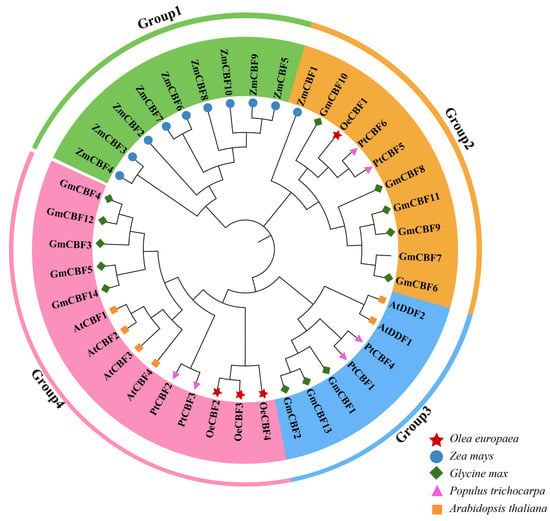
The optimal temperature for olive growth, development, and fruit yield in the Mediterranean regions is 15~25 °C [10], while the limit temperature is −12~−7 °C [38,39], which indicates that the cold tolerance is an important criterion for olive varieties to adapt to different environments. CBF transcription factors were reported to play a significant role in mediating plant responses to biotic and abiotic stresses, including high salt, drought, and low temperature [40,41]. Particularly, CBFs can enhance the resistance to cold stress in plants such as Arabidopsis [42], grape [43], eggplant [44], pomegranate [45], and maize [46], implying the potential in other species. Therefore, in this study, four CBF genes were identified from the olive genome (Figure 3a), which were distributed on three different chromosomes with isoelectric points ranging from 4.87 to 9.13 (Table 1), similar to that of the tea tree [24]. The prediction of subcellular localization indicated that OeCBF1 is primarily expressed in the nucleus, while OeCBF2, OeCBF3, and OeCBF4 are localized in the nucleus and cytoplasm (Table 1). This localization difference is similar to other plant CBF genes, such as those in rye [47]. Notably, research on CBF genes across various plants has demonstrated that nuclear-localized genes are capable of regulating cold resistance. For instance, SmICE1a in eggplant [44], ZmmICE1 in maize [46], and PpCBF3 in cold-tolerant Kentucky bluegrass [48] are all nuclear-localized and have been shown to enhance plant cold tolerance. Based on these findings, it can be hypothesized that OeCBFs may share similar functions.
Gene covariance helps explore the evolutionary linkage between genes by comparing the conservation and variability, which in turn provides potential target genes for molecular-assisted breeding, thus accelerating the process of genetic improvement [49,50]. The secondary structure composition of OeCBFs was some different in that OeCBF1 lacks a β-bridge, differing from OeCBF2, OeCBF3, and OeCBF4 (Table 2). The protein evolutionary relationship of OeCBF2, OeCBF3, and OeCBF4 was in a group excluding OeCBF1, further suggesting a closer relationship among OeCBF2, OeCBF3, and OeCBF4. CBF/DREB proteins usually contain an AP2 structural domain and a CBF signaling motif DSAWR, such as the CBF members in A. thaliana, B. napus, wheat, and tomato all contain two characteristic sequences, AP2 and DSAWR [51,52]. And there are also two very conserved amino acids, valine (V) and glutamic acid (E), in the AP2 region, which are crucial for the DNA binding ability and transcriptional activation of CBF proteins [53,54]. The four OeCBF proteins had an AP2/ERF domain (Figure 3a) and were highly conserved at the DSAWR sequence (Figure S2). Moreover, the four OeCBFs contained motif3 aliases corresponding to the highly conserved DSAWR, indicating that the OeCBFs may have complete CBF functions.
Cis-regulatory elements play crucial roles in plant growth and response to abiotic stresses and determine target gene expression patterns, including tissue specificity, stress responses, and hormone responses [55]. The promoters of the four OeCBF genes contained abundant cis-regulatory elements involved in plant growth homeostasis, development, hormonal responses, and stress responses (Figure 3c). Notably, two stress response elements were predicted, namely, the drought response element (MBS) and the low-temperature response element (LTR). Especial OeCBF2 contained LTRs, hypothesizing that OeCBF2 can respond to low-temperature stress and may be involved in the regulation of low-temperature tolerance in plants.
To understand the potential function of OeCBFs in growth and development, firstly, the tissue expression patterns of the OeCBF genes in two varieties, Picholine and Arbequina [32], were analyzed and found distinct tissue specificity with notable variance between the two species and corelated to the cold resistance of the varieties. The differential expression of genes has been correlated with the level of plant resistance. For instance, the Wcbf2 gene in wheat exhibited higher transcript levels in the resistant cultivar ‘Mironovskay 808’ than in the less resistant cultivar ‘Chinese Spring’ and that is why it was suggested as a pivotal gene for determining wheat resistance [56]. Similarly, in tea trees, the CsCBF5 gene was highly expressed in the cold-tolerant varieties ‘Shifo Cui’, ‘Shu Tea’, ‘Anhui Tea 91’, and ‘Cui Green 1’, but it was less expressed in the cold-sensitive cultivars like ‘Huang Kui’, ‘E Tea’, ‘Golden Tooth’, and ‘Nibel Yellow’. Further research confirmed that overexpression of CsCBF5 improved the cold tolerance of tea [57]. Additionally, the expression levels of CBF1, CBF2, CBF3, and CBF4 were higher in the grape variety ‘Khalili-Danedar’ than in ‘Shahroodi’, while ‘Khalili-Danedar’ is a grape variety endemic to cold regions and is known for its excellent cold tolerance [58]. In practice, Picholine has shown better resistance to low-temperature stress compared to Arbequina. The differential expression trends in OeCBF2 and OeCBF4 in Picholine and Arbequina imply that these genes may be key determinants of cold tolerance in olive trees. The upregulation of OeCBF2 and OeCBF4 under low-temperature stress (Figure 5a, c), as well as their correlation in stem and leaf tissues, further suggests the significant roles of these two genes in conferring cold resistance of olive plants. Moreover, the high correlation between the expression levels of OeCBF2 and OeCBF4 in six different tissues of both Picholine and Arbequina suggests a significant association between these two genes and the cold resistance of olive plants (Figure 5b,d).
To further elucidate the functional potential of OeCBFs in response to cold stress, Picholine and Arbequina were exposed to varying degrees of cold treatments and the transcription of OeCBFs was analyzed. We interestingly found that all OeCBFs were induced in roots, stems, and leaves under different cold conditions (Figure 6), which revealed that all OeCBFs may relate to cold stress response in olive trees. In Picholine, the OeCBFs in leaves were most sensitive to cold stress, while in Arbequina, the OeCBFs in stems were most responsive. Notably, the OeCBF2 gene showed the most prominent expression (Figure 6). Expression activity in response to stress is considered an effective predictor of functionality. For instance, the JcCBF2 gene from Jatropha was highly induced by 4 °C [59], and later found to enhance plant resistance to low temperatures [60]. Similarly, the PgCBF3 and PgCBF7 from pomegranate were significantly upregulated under cold stress, and transgenic Arabidopsis lines overexpressing PgCBF3 and PgCBF7 exhibited higher survival rates after cold treatment [45]. In melon, CmCBF4 was markedly induced by cold stress, while during the silencing of CmCBF4, the cold tolerance of melon was reduced [61]. Therefore, the induced expression of OeCBFs in response to cold stress indicates that they are functionally significant and representative of the olive’s low-temperature response.
Source link
Guanghui Hai www.mdpi.com