1. Introduction
Among cetaceans, several groups of the same species may adapt and evolve independently due to behavioral specializations that limit gene flow (e.g., [
1,
2,
3,
4]). As a result, patterns in genetic population structure are often shaped by behavioral, social, or cultural traits [
3,
5,
6,
7,
8]. Genetic diversity, correlated with intraspecific variations in spatial distribution, foraging ecology, and social structure, may lead to the emergence of ecotypes. First introduced in plants [
9], the concept of “ecotype” is complex, partly due to its interdisciplinary nature, but it has proven highly valuable for evolutionary biology and applied conservation [
10]. This complexity has led to some confusion and inconsistencies in the literature, especially regarding the criteria used to define ecotype status [
11,
12]. A commonly accepted definition is that of Lowry [
11], which is also used by Louis et al. [
3], which considers ecotypes as groups of populations that differ in genetic traits as well as ecological and/or physiological characteristics. Building on this, Stronen et al. [
10] outlined a framework to distinguish ecotypes, based on three conditions: phenotypic variations, differences in preferred habitats, and genetic distinction, which must be observed for groups of the same species to qualify as ecotypes.
Today, several marine mammal species are known to include ecotypes. Several ecotypes of killer whales (
Orcinus orca) have been described, often associated with feeding specialization. In the Northeast Pacific, resident, transient, and offshore ecotypes are distinguished by multiple independent lines of evidence [
13] based on genetic, morphological, geographical, and dietary characteristics [
12]. The differences between ecotypes are so pronounced that Morin et al. [
14] revised the taxonomy of two eastern North Pacific ecotypes, which are now classified as distinct subspecies:
O. orca ater (resident killer whale) and
O. orca rectipinnus (Bigg’s killer whale, also known as the transient ecotype). The Antillean manatee (
Trichechus manatus manatus) also exhibits distinct ecotypes, influenced by its diverse habitats. Comparisons of body condition indices revealed differences between manatees in freshwater ecosystems and those in coastal and marine areas. These variations suggest that the subspecies comprises at least two ecotypes—riverine and coastal—each facing different fitness tradeoffs due to environmental and resource limitations [
15].
The common bottlenose dolphin (
Tursiops truncatus) is another example of a marine mammal species exhibiting highly complex intraspecific diversity.
T. truncatus is a cosmopolitan species found in tropical to temperate waters, both in coastal and estuarine ecosystems as well as in the open ocean. Currently, four subspecies of
T. truncatus are officially recognized [
16]. In addition, two ecotypes of
T. truncatus have been described over the past several decades [
3,
17,
18,
19,
20,
21,
22,
23,
24,
25]. Often referred to as coastal and oceanic, the ecotypes of
T. truncatus have been distinguished based on various independent lines of evidence, which do not always meet all three criteria defined by Stronen et al. [
10]: hematological profiles [
26], parasite load [
27], skull morphology [
1,
28], genetic structure [
4,
29,
30,
31], and morphology (see
Table S1). In the Atlantic, in terms of morphology, the oceanic ecotype of
T. truncatus is generally larger (total length, skull length, and internal nostril width), darker, has a more falcate dorsal fin, and has a shorter rostrum than the coastal ecotype [
29,
32,
33,
34].
T. truncatus ecotypes seem to be almost ubiquitous, having been widely detected from the Atlantic to the Pacific. However, there are counterexamples. For instance,
T. truncatus ecotypes were not detected in the Azores by Quérouil et al. [
35]. In the northern Caribbean Sea, Caballero et al. [
36] studied the genetic diversity of
T. truncatus in a region encompassing the Gulf of Mexico, Honduras, Colombia, the US Virgin Islands, Puerto Rico, Jamaica, Cuba, and the Bahamas. Mitochondrial DNA polymorphisms demonstrated the regional presence of at least two genetically differentiated forms of
T. truncatus, the coastal ecotype and the worldwide distributed form, which may correspond to the oceanic ecotype. These results have been confirmed in other regions of the Caribbean, such as La Guajira (Colombia), Panamá (Bocas del Toro), and Costa Rica [
37,
38].
Whether the
T. truncatus ecotypes are on distinct evolutionary trajectories remains an open question [
1]. In fact, ecotypes may coincide with distinct taxonomic units, such as subspecies or species [
39]. To formulate a taxonomic hypothesis in cetaceans, such as delimiting subspecies, authors must specify the indices used as the basis for the hypothesis and those employed to stratify the data [
13]. These indices often include discrepancies in geographic distribution, morphology, ecology, or acoustics, facilitating the review of several pertinent independent lines of evidence [
13,
17]. The framework proposed by Stronen et al. [
10] aligns with this approach for ecotype delimitations. The
T. erebennus species, the most recently recognized species of the genus, was first identified as the coastal ecotype of
T. truncatus in the Northwest Atlantic [
1]. Coastal and oceanic ecotypes of
T. truncatus exhibit marked genetic differentiation, which can sometimes be greater than that observed between (sub)species (e.g., [
18]).
Distinguishing ecotypes in T. truncatus is therefore of fundamental interest for understanding the genetic diversity of this highly mobile cetacean species with an extensive home range. It is equally critical for the effective management and conservation of the species, especially where local concerns are known.
Given its cosmopolitan distribution,
T. truncatus is globally classified as Least Concern (LC) by the IUCN. However, several local populations are threatened, as demonstrated, for instance, in New Zealand [
40]. Additionally, other groups, which appear to be highly isolated, of low abundance, and with restricted home ranges, require intensive monitoring. Notable examples include populations around the island of Sein in Brittany [
41] and in the Bay of Setúbal in Portugal [
42]. While this species has been extensively studied in some regions, targeted research is therefore strongly needed in other areas to address knowledge gaps and inform effective conservation strategies.
The Guadeloupe Archipelago, located at the heart of the Agoa Sanctuary—a vast marine protected area covering the entire French exclusive economic zone of the Lesser Antilles and dedicated to the protection and conservation of marine mammals—serves as a typical example of a place where comprehensive studies of
T. truncatus ecotypes are essential. A major part of the present knowledge about the species comes from the surveys conducted since 2011 by the “
Observatoire des Mammifères Marins de l’Archipel Guadeloupéen” (OMMAG), an NGO dedicated to citizen science projects [
43]. Over a decade of photo-identification monitoring has revealed the presence of two distinct morphotypes (groups of individuals from the same species sharing specific morphological characteristics) of
T. truncatus in the archipelago. However, whether these morphotypes correspond to distinct coastal and oceanic ecotypes, as described in other regions, remains uncertain.
To address this question, this study aims to determine whether the two observed morphotypes of
T. truncatus correspond to distinct coastal and oceanic ecotypes. To achieve this, we (i) characterized the morphological differences between the two morphotypes, (ii) looked for the existence of genetic variation in
T. truncatus individuals stranded around Guadeloupe in the last ten years and correlated these variations with ecotype-specific DNA data published by others in Genbank, and (iii) modeled the habitat of both morphotypes around Guadeloupe in order to detect potential differences in preferential habitats. Additionally, based on previous studies analyzing the maritime traffic around Guadeloupe [
44,
45], we highlighted differential exposure risks for the two ecotypes of
T. truncatus around Guadeloupe.
This study is the first to investigate the presence of coastal and oceanic T. truncatus ecotypes within the Agoa Sanctuary, providing novel insights into their morphology, genetics, and habitat preferences. By identifying ecotype-specific risks, we emphasize the need to integrate these findings into conservation strategies tailored to the Guadeloupe Archipelago. To implement these objectives, we employed an integrated approach combining photographic, genetic, and ecological data analysis.
4. Discussion
The occurrence of the two ecotypes of
T. truncatus, coastal and oceanic, has been documented across a wide geographical range. However, the situation is not uniform and appears to be more complex to decipher in certain geographical areas such as the Azores and Madeira [
35], the Pacific Ocean, or the North-East Atlantic (NEA) [
3,
33,
80]. The three criteria defined by Stronen et al. [
10] (i.e., phenotypic variation, differences in preferred habitats, and genetic differentiation) provide a solid basis for recognizing and characterizing the presence of both ecotypes in a given location. Even if this basis for ecotype identification is not standardized across studies, with some relying solely on ecological and morphological parameters [
20] or genetic analyses (e.g., [
41]), the failure to detect both ecotypes in some places seems to be linked more to a real heterogeneity in their distribution than to technical issues of identification.
Here, we sought to determine whether the two morphotypes of
T. truncatus recently detected by citizen science observers around the Guadeloupe archipelago met the three criteria of Stronen et al. [
10] and could therefore be considered coastal and oceanic eco-types, whose existence would thus be proven locally for the first time.
4.1. Two Distinct Morphotypes with Different Preferred Habitats
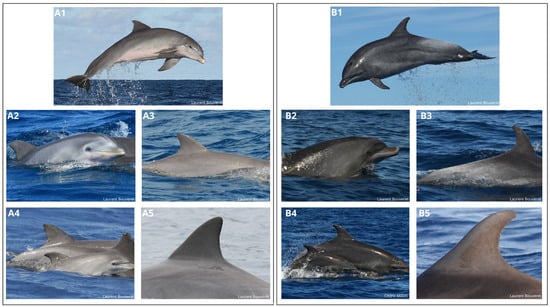
Photo-analyses revealed the presence of two distinct morphotypes of
T. truncatus in Guadeloupe, the coastal morphotype and the oceanic morphotype, which are clearly evocative of the general morphologies of the two Atlantic ecotypes [
1,
28,
34].
Analysis of spatial distribution patterns showed that the two morphotypes in Guadeloupe are distributed in space in a similar but not identical way, which is generally expected for closely related taxonomic groups [
61]. The results of the habitat modeling confirmed these findings and specified the preferred habitats of each morphotype. The preferred habitat of the coastal morphotype was found to be mainly near the coast, while that of the oceanic morphotype was distributed further offshore.
In the Atlantic, significant habitat differences between the two ecotypes of
T. truncatus are well recognized. The coastal ecotype was mainly concentrated in areas of lower bathymetry, whereas the oceanic ecotype had a wider spatial distribution associated with a more flexible use of its habitat (e.g., [
34,
81]). Areas of partial overlap between coastal and oceanic ecotypes in NWA and SWA have also been demonstrated (e.g., [
34,
82]), similar to the results of this study. In the Gulf of Mexico, where such overlap exists, it has been hypothesized that the distribution of the oceanic ecotype in both neritic and oceanic habitats could be one of the reasons for this overlap [
36].
In the marine environment, local variations in habitat characteristics can drive the development of specialized ecological niches [
29]. In cetaceans, ecological niches appear to be linked to water temperature and depth, as well as any factors affecting the distribution and abundance of their prey [
37,
81]. Indeed, habitat differences appear to influence the feeding behaviour of
T. truncatus [
31,
83,
84]. In NWA, it was suggested that some of the differences between the two ecotypes were probably associated with distinct feeding ecologies directly related to their habitat [
1]. More broadly, ecological opportunities for specialization may have been the main driver of ecological, morphological, and genetic divergence in
T. truncatus [
81].
4.2. Two Distinct Guadeloupean Genetic Groups of T. truncatus Correlated to Caribbean Ecotypes
Analysis of the polymorphisms in the mitochondrial DNA of T. truncatus in Guadeloupe identified two distinct genetic groups strongly separated by at least 19 nucleotide differences out of 676 bp.
When compared to larger geographic scale DNA sequence data (i.e., Caribbean data, and then whole Atlantic data), these two groups presented obvious sequence similarities with the coastal T. truncatus ecotype in the case of one, and with the oceanic ecotype in the case of the other.
It can be noted that similar results were obtained by Rodriguez-Ferrer et al. [
85] in Puerto Rico.
In Guadeloupe, the haplotype diversity and nucleotide diversity were markedly different between the two groups. The coastal group (Group A) exhibited lower haplotype diversity (HdA = 0.476) and nucleotide diversity (πA = 0.019) compared to the oceanic group (Group B), which showed a haplotype diversity (HdB = 1.000) and nucleotide diversity (πB = 0.261) values much higher. Although this observation should be interpreted in the light of the small number of samples (
n = 7 and
n = 4, respectively), these values were similar to those determined in groups of coastal
T. truncatus in the NWA [
1], SWA [
2], and NEA [
3]. The observed differences in genetic diversity between the coastal and oceanic ecotypes in Guadeloupe are likely shaped by historical demographic events, ecological factors such as habitat size and connectivity, and their respective mobility and gene flow.
Differentiation indices between the two ecotypes in Caribbean
T. truncatus, including the new data from Guadeloupe, presented high values reflecting very strong genetic differentiations between the two ecotypes. The
FST and
ΦST values determined were very close to those calculated by Costa et al. [
1] between the two ecotypes in the NWA (this study,
FST = 0.170 and
ΦST = 0.748; Costa et al. [
1],
FST = 0.21 and
ΦST = 0.71).
For oceanic ecotypes, geographical proximity seems to have less influence on genetic closeness, and Guadeloupean oceanic
T. truncatus appears to be as close to the Caribbean oceanics as they are to those from the Mediterranean and the NEA. Generally speaking, in the Atlantic, oceanic
T. truncatus appear to form a single large genetic group, supporting the designation of the oceanic ecotype of
T. truncatus as ‘Worldwide distributed form’ (WDF), proposed by Tezanos-Pinto et al. [
80]. Similar results have recently been highlighted by Gómez-Lobo et al. [
86], who demonstrated high connectivity with the Northeast Atlantic via mitochondrial variation in
T. truncatus from the Canary Islands.
The Atlantic basin-scale network highlighted that the genetic divergence between the two ecotypes of
T. truncatus was much more marked in the Northwest Atlantic than in the Northeast Atlantic, corresponding clearly to an older separation. This finding is consistent with the results of Louis et al. [
4], who showed that the oldest divergence between oceanic and coastal ecotypes occurred in the Northwest Atlantic (estimated at 80,000 BP) and the most recent in the Northeast Atlantic (estimated at 12,000 BP). This information supports the hypothesis that coastal Caribbean
T. truncatus emerged from Atlantic oceanic ones around 80,000 years ago [
4]. This emergence is likely to have occurred under the influence of genetic drift and possible reproductive isolation, leading to the strong genetic differentiation observed nowadays between the two ecotypes in the Northwest Atlantic. Whether they are on different evolutionary trajectories remains to be seen.
4.3. Integration of Genetic, Habitat, and Morphological Data to Distinguish Two Distinct Ecotypes with Different Management Implications
The integration of genetic, habitat, and morphological data (
Table S4) provides a robust framework for identifying two distinct ecotypes [
10] of
T. truncatus within the Guadeloupe Archipelago. These three independent lines of evidence—distinct genetic groups, different morphologies, and their separate habitat use—allowed us to distinguish the morphotypes of
T. truncatus in Guadeloupe as two distinct ecotypes.
Genetic analysis revealed two distinct genetic groups in Guadeloupe: the coastal ecotype exhibited low genetic diversity, high site fidelity, and apparent genetic isolation from neighboring populations, while the oceanic ecotype showed higher genetic diversity and presumably greater gene flow. Habitat modeling corroborated these findings by demonstrating that the two morphotypes do not occupy the same habitats. The coastal morphotype is primarily found in nearshore areas, whereas the oceanic morphotype prefers deeper, offshore habitats, highlighting their different ecological requirements. Morphological differences between the two groups—such as body size, fin shape, and coloration—further distinguish them and are likely to be linked to their habitat preferences and feeding behaviors. Together, these independent lines of evidence confirmed the ecotype status of T. truncatus in Guadeloupe, emphasizing the need for targeted conservation efforts that address the specific requirements and threats facing each ecotype.
The marked differentiation and the probable limited gene flow between the two sympatric
T. truncatus ecotypes around Guadeloupe lead to concerns in terms of conservation, especially about possible differences of interactions with human activities and the need for tailored management strategies. Coastal populations generally exhibit a higher level of site fidelity, low abundance, and are genetically isolated from other neighboring populations (e.g., [
37,
41,
87]). Because of their adaptations to local conditions, habitat degradation could further affect coastal populations, which are vulnerable to rapid changes in their habitat, particularly those caused by human activities (e.g., [
88]). In Guadeloupe, the coastal ecotype has a very small population (around 30 individuals, according to photo-identification data), which raises questions about the impact of anthropogenic activities in terms of disturbance or pollution [
89]. This ecotype’s dependence on specific habitats, such as the Petit Cul-de-Sac Marin, highlights the need for protective zoning measures, such as time-area restrictions to reduce disturbance from maritime traffic, as well as actions to limit pollution and noise in these critical habitats. Long-term monitoring of population trends and genetic diversity is essential to ensure the resilience of this ecotype.
The oceanic ecotype raises fewer concerns
a priori because of its belonging to a WDF, because of its greater abundance (550 different individuals estimated by photo-identification recapture in the Guadeloupean waters), and because of its suspected greater adaptability. But it could be exposed to specific risks at local to regional scales. It is, for instance, more exposed to accidental capture by fishing gear and even targeted whaling in Saint Vincent and the Grenadines [
90].
The environmental impacts of maritime traffic, including specifically on cetaceans, are well documented (e.g., [
44,
91,
92]). Several studies have reported short-term behavioral responses of
T. truncatus exposed to shipping traffic, such as an increase in their dive time [
93] or changes in their breathing and surfacing patterns [
94,
95]. Allen et al. [
96] showed in Florida that
T. truncatus decreased their use of feeding sites during periods of high maritime traffic density. Russell et al. [
97] defined ‘high-risk management zones’ as geographical areas where a high density of cetaceans and intense maritime traffic converge. In view of the results obtained in this study, the two ecotypes of
T. truncatus in Guadeloupe are exposed to intense maritime traffic in at least part of their habitats. The oceanic ecotype appears to have more hot spots of exposure, which may be explained by its wider ecological niche. The coastal ecotype is most exposed in the Petit Cul-de-Sac Marin, which is a compulsory passage zone for all ships transiting to the island’s port area. Generally speaking, in Guadeloupe,
T. truncatus are particularly exposed to maritime traffic in the Côte-sous-le-vent (the west coast of the island), which could be considered a ‘high-risk management zone’ for the species.
Our findings underscore the importance of tailoring conservation strategies to the distinct needs of these ecotypes. The coastal ecotype, with its small and localized population, requires intensive habitat protection and disturbance mitigation, while the oceanic ecotype would benefit from regional collaborations addressing broader-scale threats. By identifying these management priorities, this study contributes to the development of evidence-based conservation strategies for T. truncatus in Guadeloupe.