Author Contributions
Conceptualization, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Methodology, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Investigation, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Validation, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Visualization, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Writing—original draft preparation, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. Writing—review and editing, I.P., R.P., L.C., B.B., I.S., G.P., A.D., R.R., A.O., A.G., L.D., A.M., R.M. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
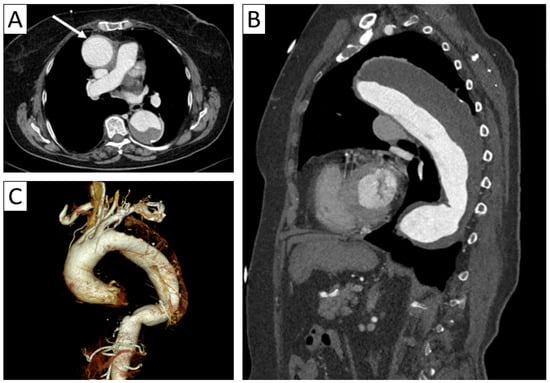
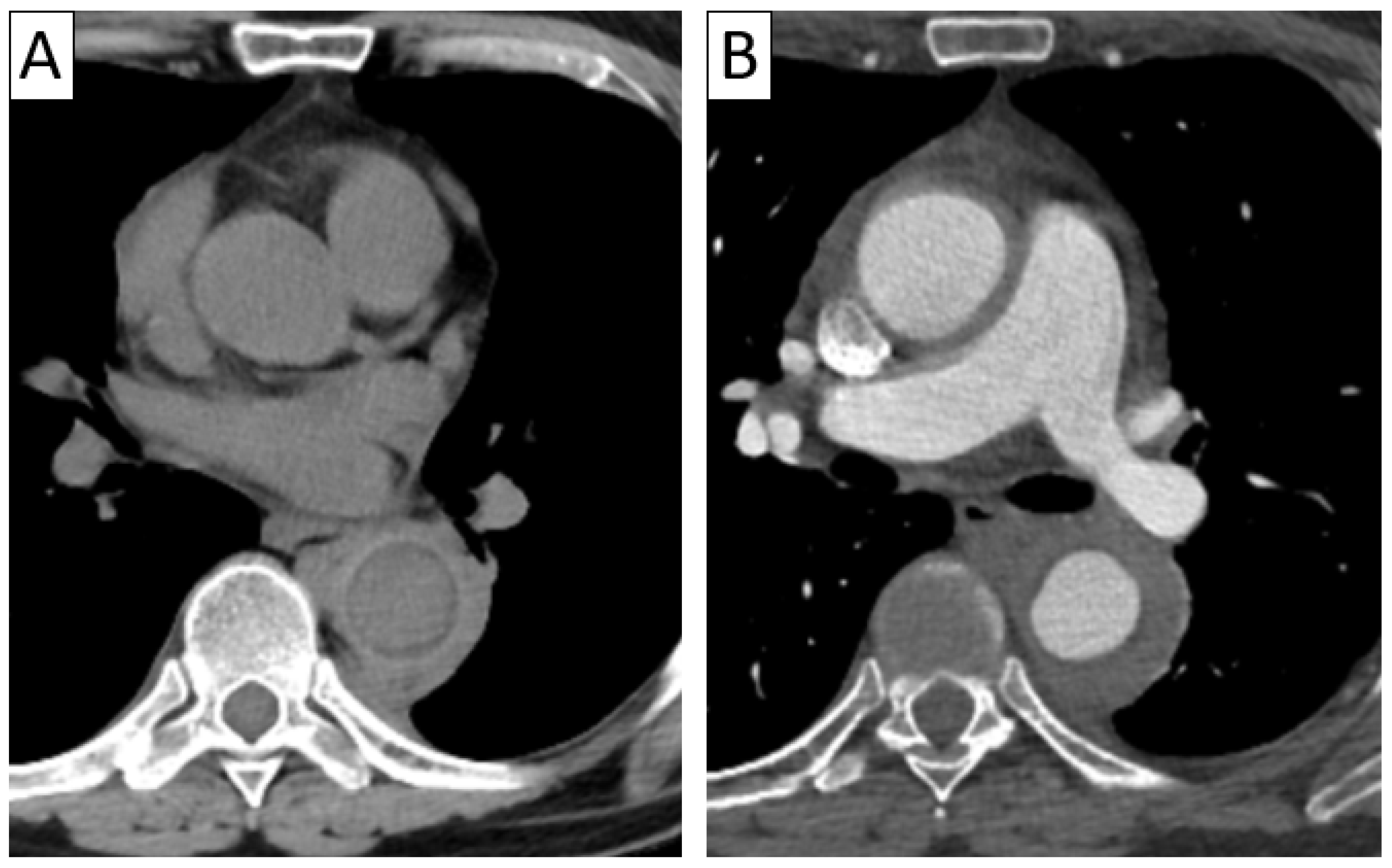
An 80-year-old female patient with giant cell arteritis and rheumatoid arthritis. Axial thoracic CTA (A) with sagittal reformatting (B) and 3D volume rendering (C) demonstrated minimal thickening of the aortic wall (arrow in (A)) and a fusiform aneurysm of the ascending and descending thoracic aorta with parietal thrombosis.
Figure 1.
An 80-year-old female patient with giant cell arteritis and rheumatoid arthritis. Axial thoracic CTA (A) with sagittal reformatting (B) and 3D volume rendering (C) demonstrated minimal thickening of the aortic wall (arrow in (A)) and a fusiform aneurysm of the ascending and descending thoracic aorta with parietal thrombosis.
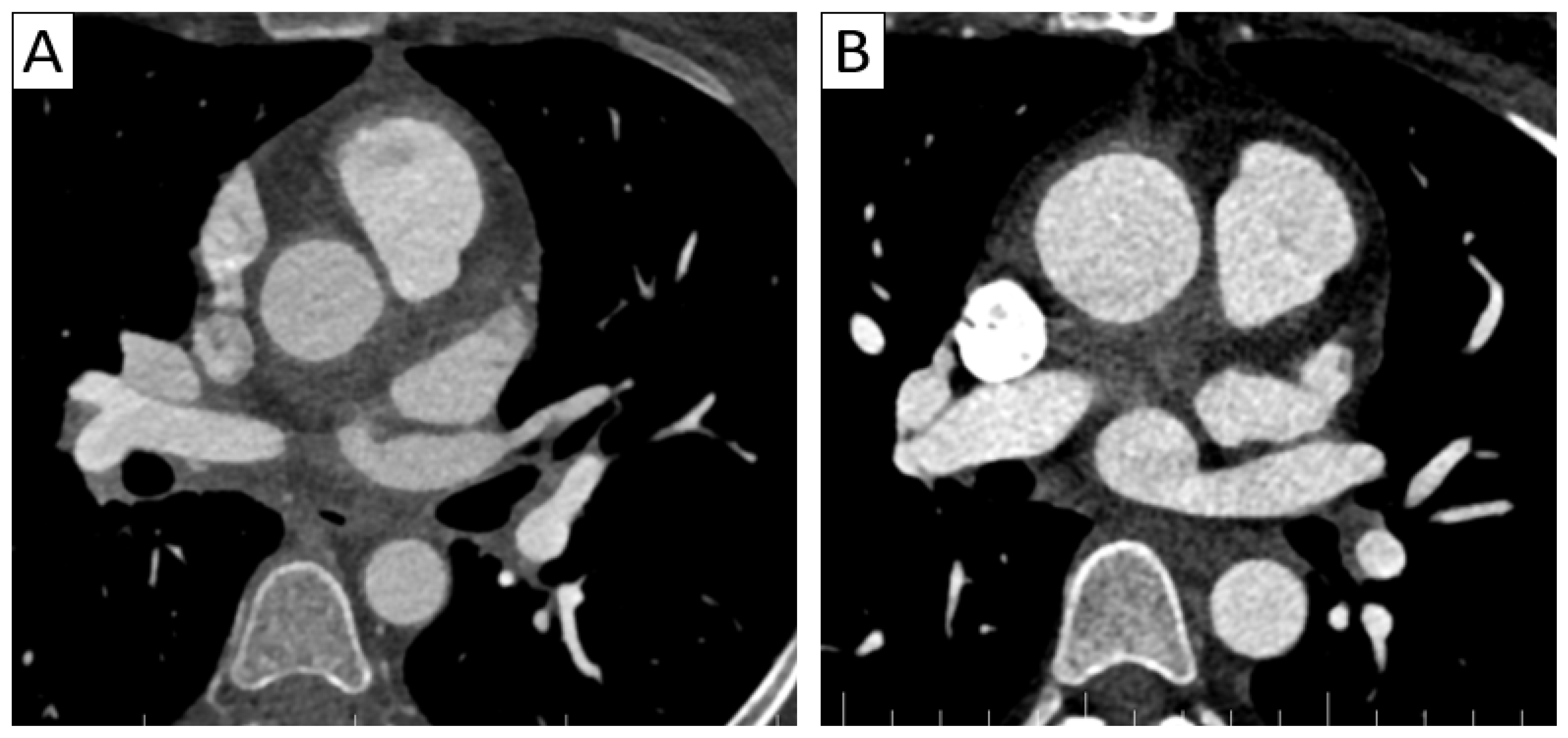
(A) A 37-year-old patient with Takayasu arteritis presenting with unstable angina. Axial thoracic CTA demonstrated aortic wall thickening and periaortic infiltration, as well as parietal inflammation of the supra-aortic trunks and coronary arteries, with myocardial infarction in the left anterior descending coronary artery territory. (B) A 35-year-old patient with Takayasu arteritis presenting with severe aortic insufficiency—aortitis was suspicioned intraoperatively during valvular replacement. Axial thoracic CTA demonstrated parietal thickening of the ascending aorta and periaortic infiltration.
Figure 2.
(A) A 37-year-old patient with Takayasu arteritis presenting with unstable angina. Axial thoracic CTA demonstrated aortic wall thickening and periaortic infiltration, as well as parietal inflammation of the supra-aortic trunks and coronary arteries, with myocardial infarction in the left anterior descending coronary artery territory. (B) A 35-year-old patient with Takayasu arteritis presenting with severe aortic insufficiency—aortitis was suspicioned intraoperatively during valvular replacement. Axial thoracic CTA demonstrated parietal thickening of the ascending aorta and periaortic infiltration.
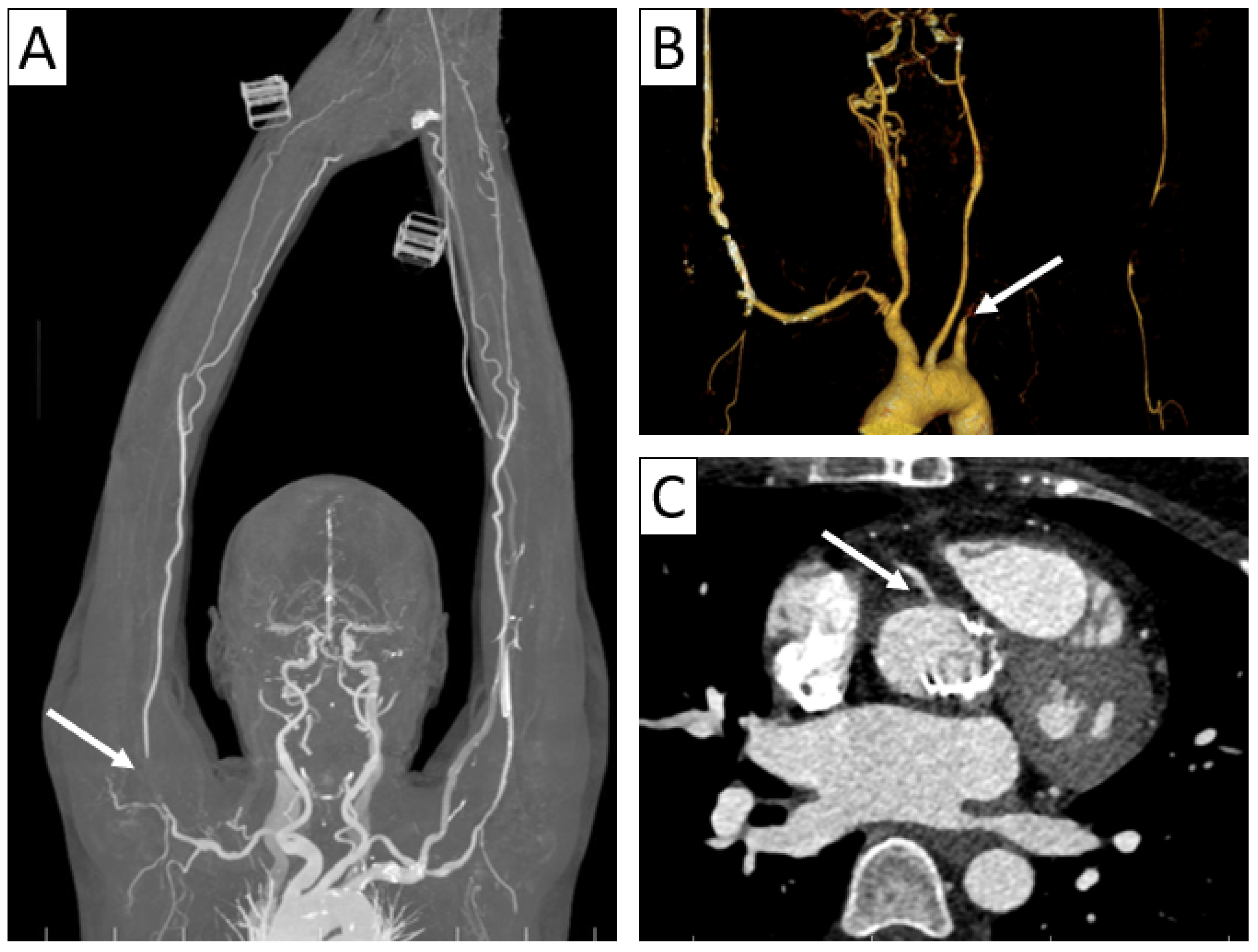
Complications of vasculitis—stenoses and occlusions. (A) Maximum intensity projection (MIP) of 3D-CTA showed brachial sub-occlusion (arrow) in a patient with parietal thickening of up to 4 mm in the supra-aortic trunks with extension to the axillary and brachial arteries. (B) Supra-aortic trunk CTA 3D volume rendering in a 38-year-old patient with TAK and Behcet’s disease overlap showed occlusion of the left subclavian and axillary arteries (arrow) with weak brachial artery distal reperfusion. (C) Axial cardiac CT in a 35-year-old patient with TAK revealing aortic wall thickening extending to the right coronary artery ostium with minor stenosis (arrow).
Figure 3.
Complications of vasculitis—stenoses and occlusions. (A) Maximum intensity projection (MIP) of 3D-CTA showed brachial sub-occlusion (arrow) in a patient with parietal thickening of up to 4 mm in the supra-aortic trunks with extension to the axillary and brachial arteries. (B) Supra-aortic trunk CTA 3D volume rendering in a 38-year-old patient with TAK and Behcet’s disease overlap showed occlusion of the left subclavian and axillary arteries (arrow) with weak brachial artery distal reperfusion. (C) Axial cardiac CT in a 35-year-old patient with TAK revealing aortic wall thickening extending to the right coronary artery ostium with minor stenosis (arrow).
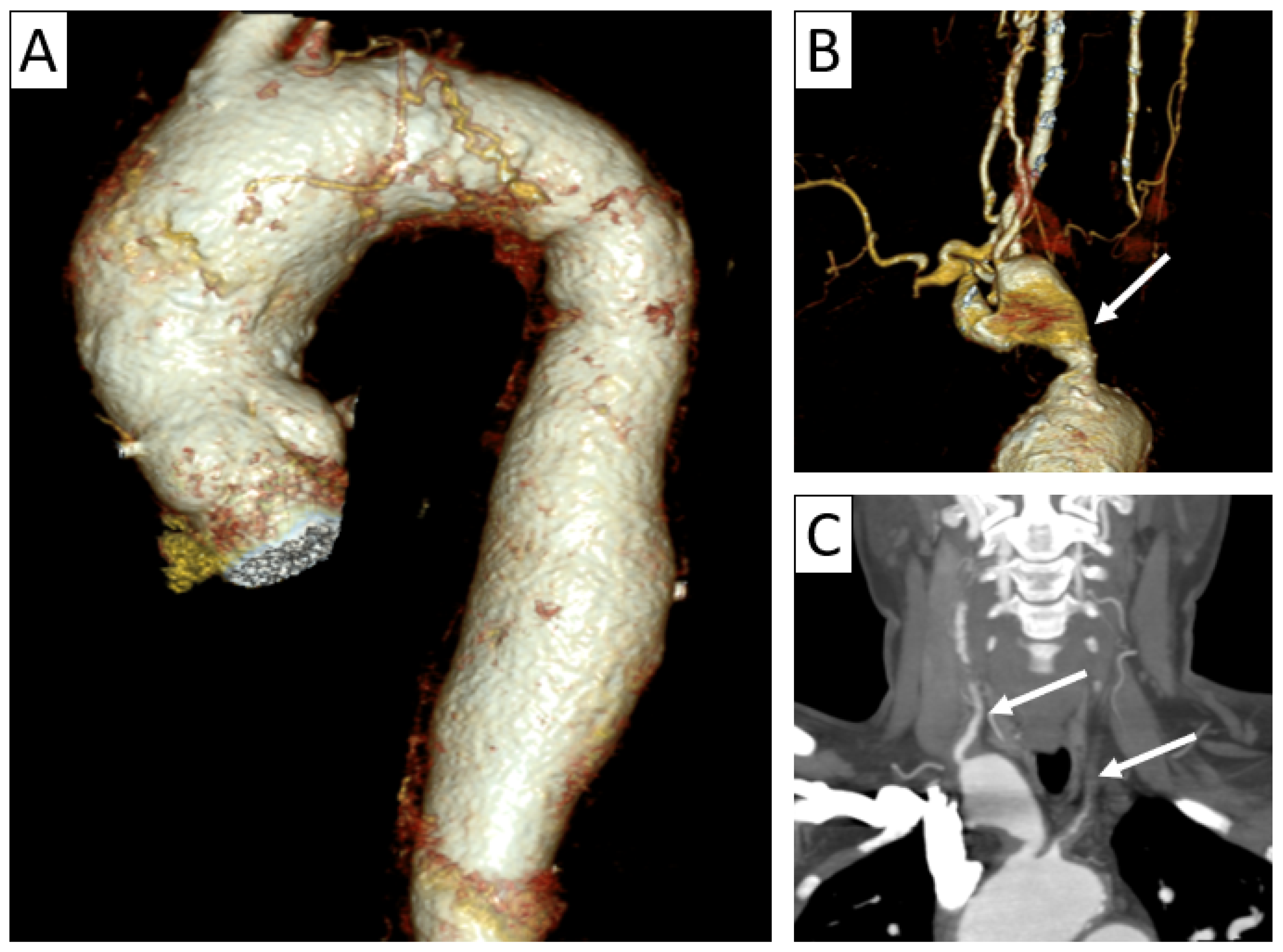
Complications of vasculitis—stenoses and aneurysms. Thoracic and supra-aortic trunk CTA with 3D volume rendering (A,B) and coronal reformatting (C) in a 41-year-old patient with TAK showing ectasias of the ascending and descending thoracic aorta (A), aneurysm of brachiocephalic artery (arrow in (B)) and stenoses of the common carotid arteries (arrows in (C)), as well as subclavian arteries.
Figure 4.
Complications of vasculitis—stenoses and aneurysms. Thoracic and supra-aortic trunk CTA with 3D volume rendering (A,B) and coronal reformatting (C) in a 41-year-old patient with TAK showing ectasias of the ascending and descending thoracic aorta (A), aneurysm of brachiocephalic artery (arrow in (B)) and stenoses of the common carotid arteries (arrows in (C)), as well as subclavian arteries.
Contrast-enhanced axial thoracic CTA in a patient with TAK showing a highly attenuating thickened wall of the descending aorta on unenhanced scan (A) and the “double ring” sign on venous-phase imaging (B): a hypodense internal ring (intima) surrounded by an enhancing outer ring (media and adventitia).
Figure 5.
Contrast-enhanced axial thoracic CTA in a patient with TAK showing a highly attenuating thickened wall of the descending aorta on unenhanced scan (A) and the “double ring” sign on venous-phase imaging (B): a hypodense internal ring (intima) surrounded by an enhancing outer ring (media and adventitia).
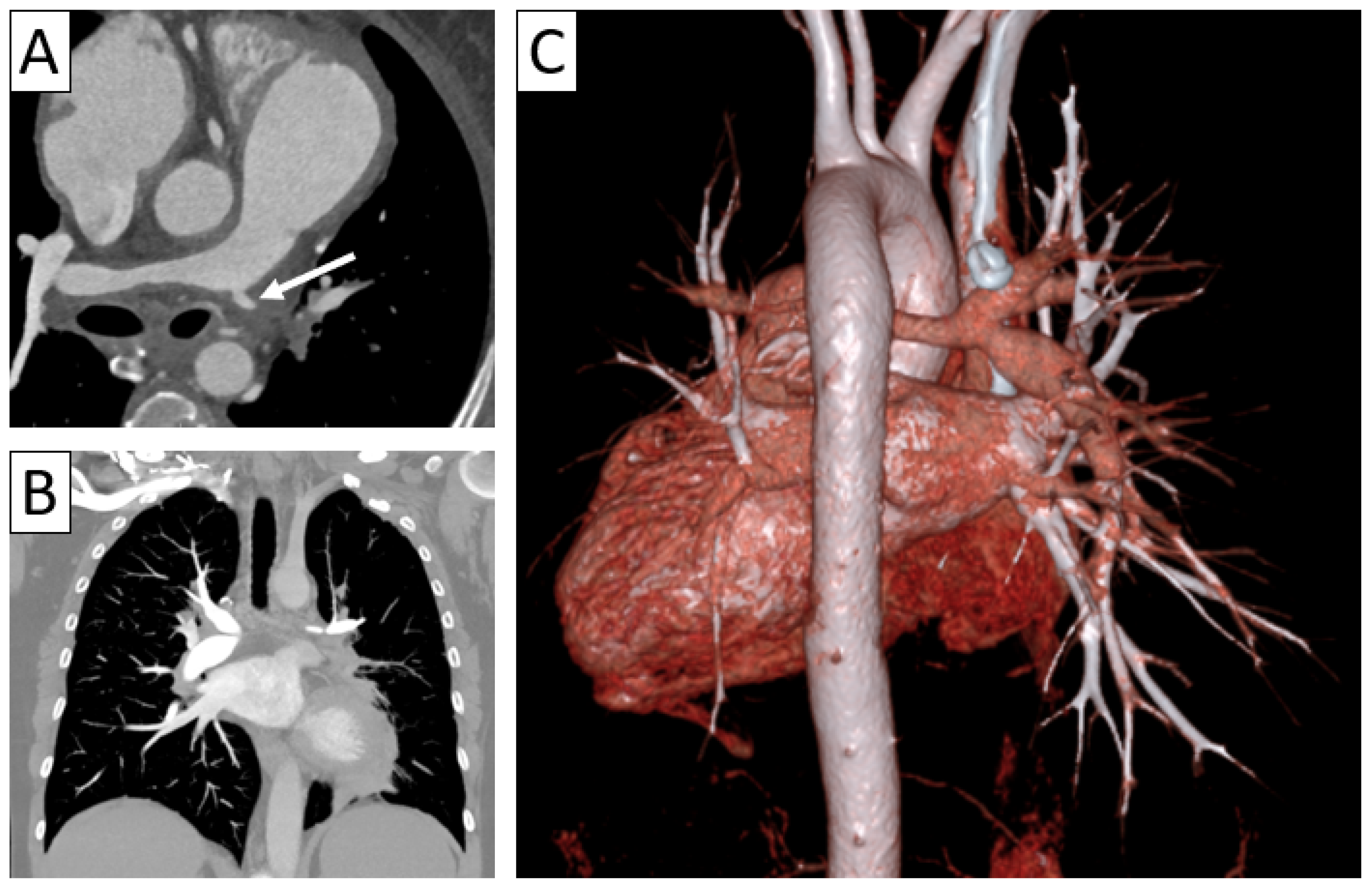
A 48-year-old female patient with Behcet’s disease presenting with oral aphthous ulcers and dyspnea. (A) Axial thoracic CTA showed diffuse thickening of the pulmonary trunk and arteries with left pulmonary artery stenosis (arrow) with extension to the lobar, segmental, and subsegmental left pulmonary arteries and subsequent vascular paucity and reduced volume of the left lung seen on coronal reformatting (B) and 3D volume rendering (C).
Figure 6.
A 48-year-old female patient with Behcet’s disease presenting with oral aphthous ulcers and dyspnea. (A) Axial thoracic CTA showed diffuse thickening of the pulmonary trunk and arteries with left pulmonary artery stenosis (arrow) with extension to the lobar, segmental, and subsegmental left pulmonary arteries and subsequent vascular paucity and reduced volume of the left lung seen on coronal reformatting (B) and 3D volume rendering (C).
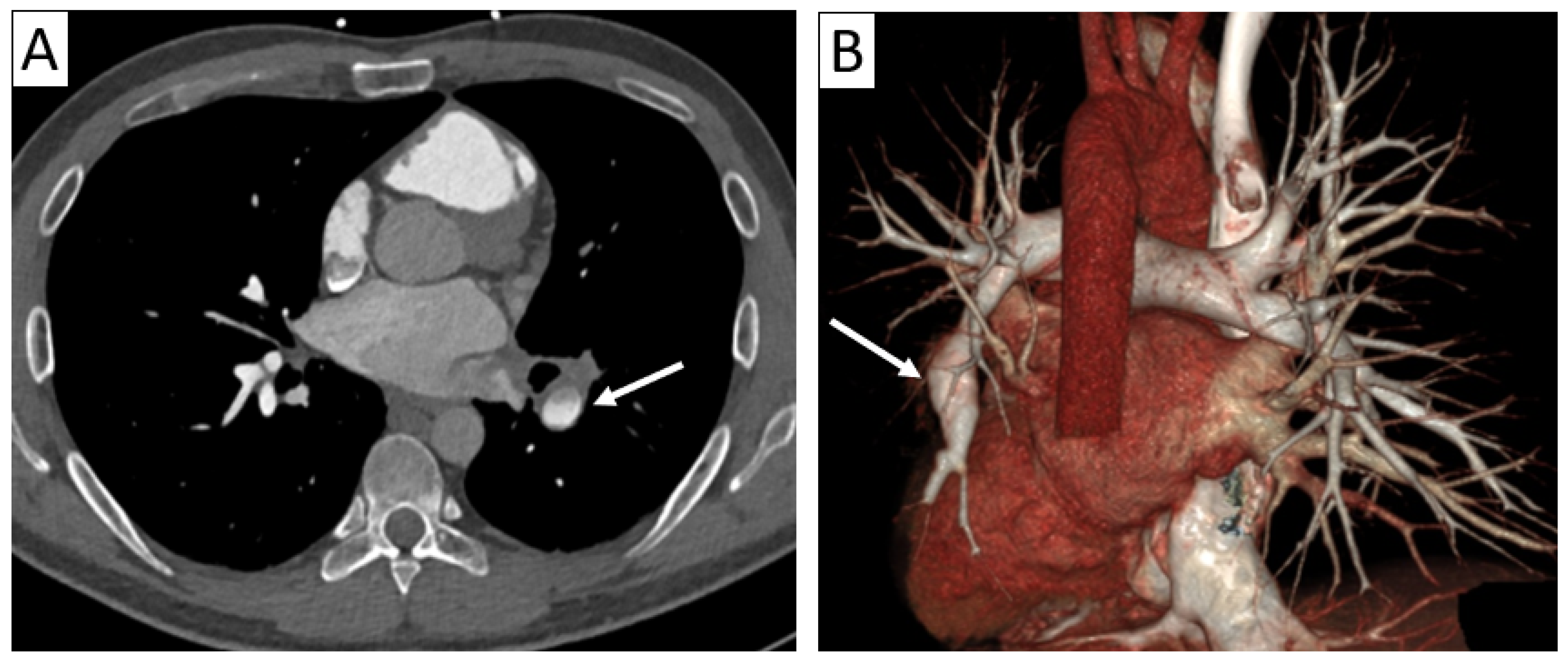
A 36-year-old male patient diagnosed with Hughes–Stovin syndrome presenting with hemoptysis and oro-genital aphthous ulcers with recurrent thrombotic events (inferior vena cava and renal veins). Axial thoracic CTA (A) with 3D-volume rendering (B) revealed a partially thrombosed left inferior lobar pulmonary artery aneurysm (arrows).
Figure 7.
A 36-year-old male patient diagnosed with Hughes–Stovin syndrome presenting with hemoptysis and oro-genital aphthous ulcers with recurrent thrombotic events (inferior vena cava and renal veins). Axial thoracic CTA (A) with 3D-volume rendering (B) revealed a partially thrombosed left inferior lobar pulmonary artery aneurysm (arrows).
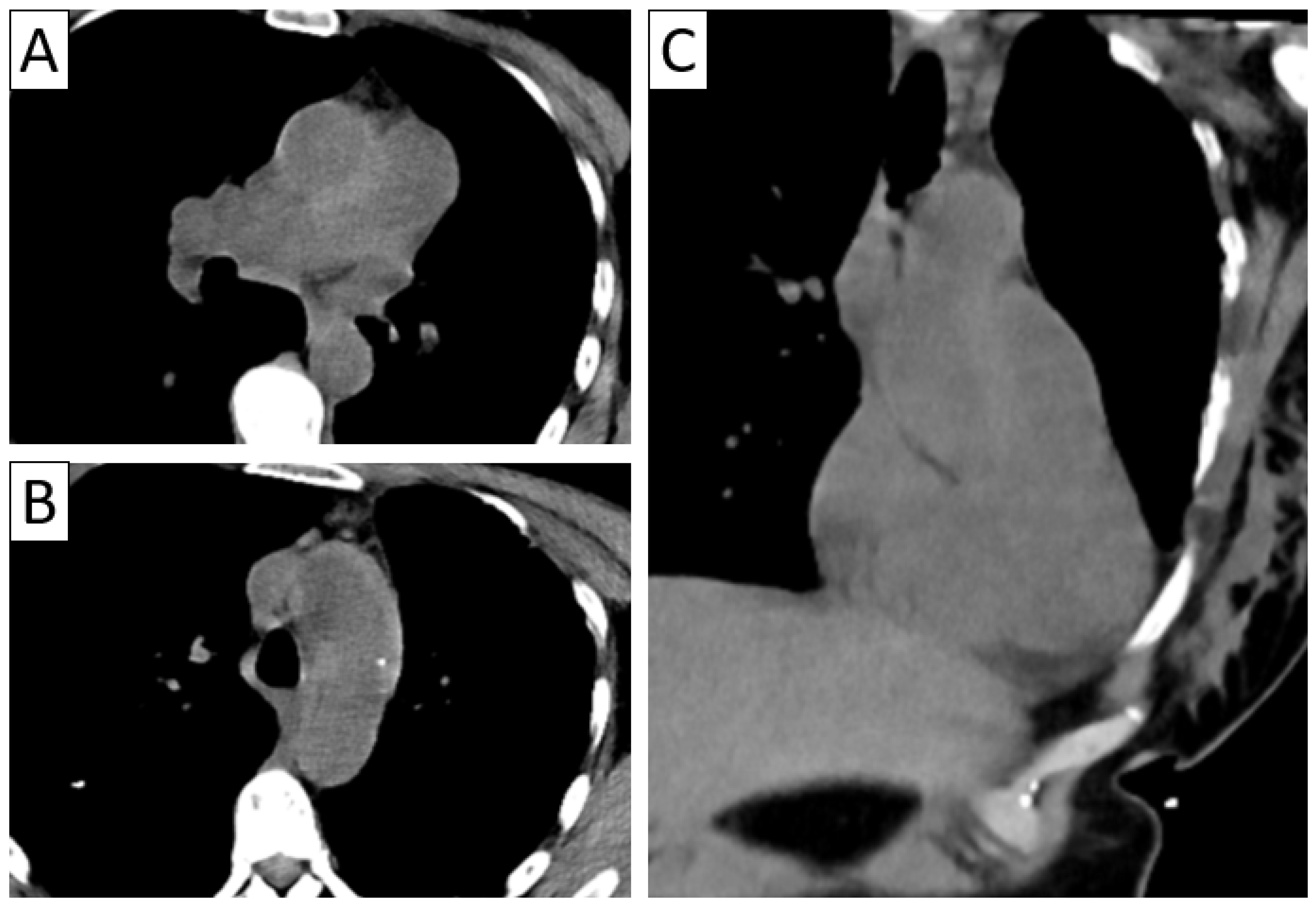
Differential diagnosis of vasculitis—intramural hematoma and pulmonary trunk sarcoma. Patient with sudden severe chest pain—unenhanced axial thoracic CT (A,B) with coronal reformatting (C) revealed arterial mural hyperdensities along the ascending aorta and pulmonary trunk suggestive of intramural hematoma; intraoperatively, suspicion of pulmonary trunk sarcoma was raised; histopathological result confirmed granulomatous necrotizing vasculitis.
Figure 8.
Differential diagnosis of vasculitis—intramural hematoma and pulmonary trunk sarcoma. Patient with sudden severe chest pain—unenhanced axial thoracic CT (A,B) with coronal reformatting (C) revealed arterial mural hyperdensities along the ascending aorta and pulmonary trunk suggestive of intramural hematoma; intraoperatively, suspicion of pulmonary trunk sarcoma was raised; histopathological result confirmed granulomatous necrotizing vasculitis.
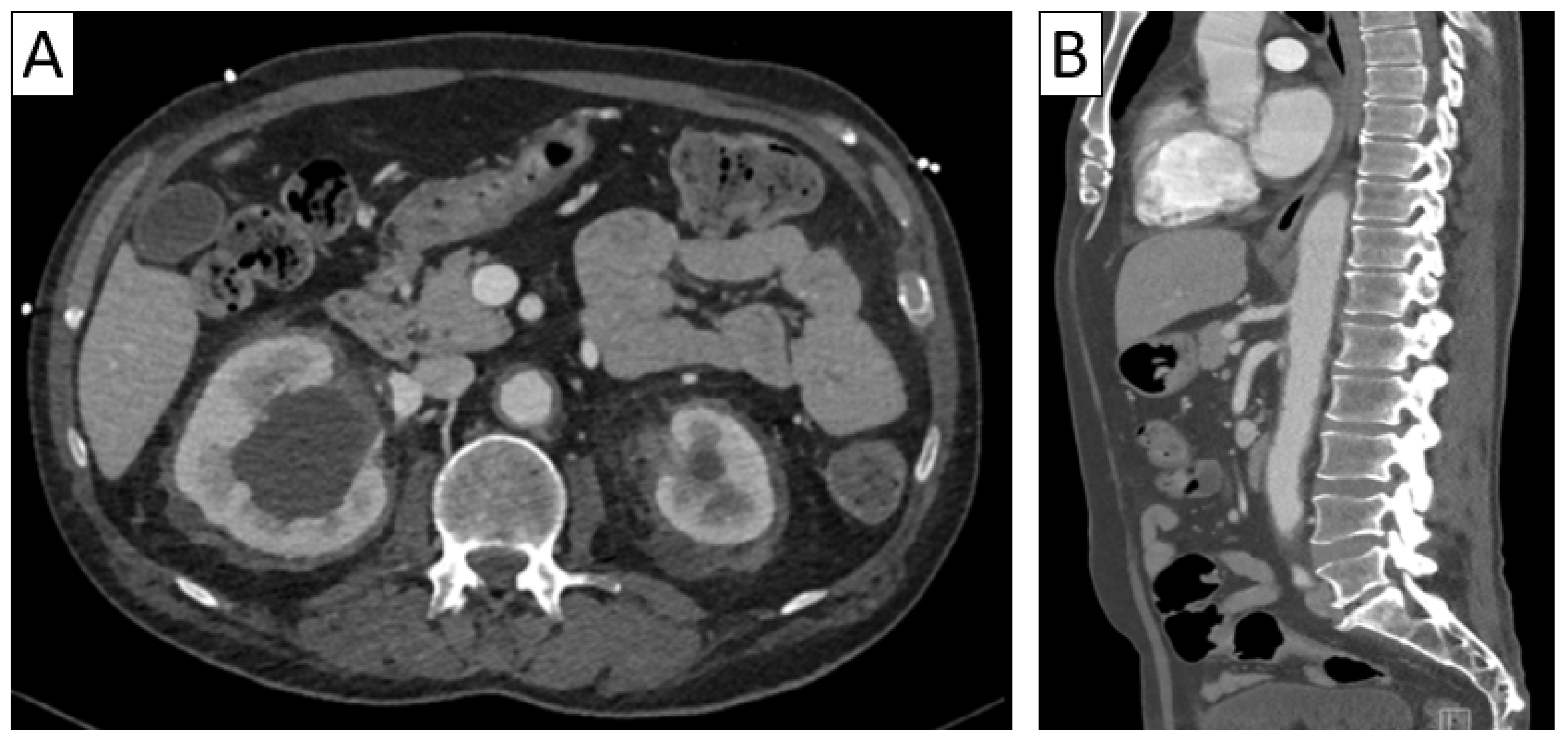
Differential diagnosis of vasculitis—Erdheim–Chester syndrome. Axial abdominal CT (A) with sagittal reformatting (B) showing diffuse retroperitoneal periaortic and perirenal tissue infiltration (“coated aorta” sign and “hairy kidneys”) in a patient with multiorgan affection (cerebral, osseous, renal, and pulmonary).
Figure 9.
Differential diagnosis of vasculitis—Erdheim–Chester syndrome. Axial abdominal CT (A) with sagittal reformatting (B) showing diffuse retroperitoneal periaortic and perirenal tissue infiltration (“coated aorta” sign and “hairy kidneys”) in a patient with multiorgan affection (cerebral, osseous, renal, and pulmonary).
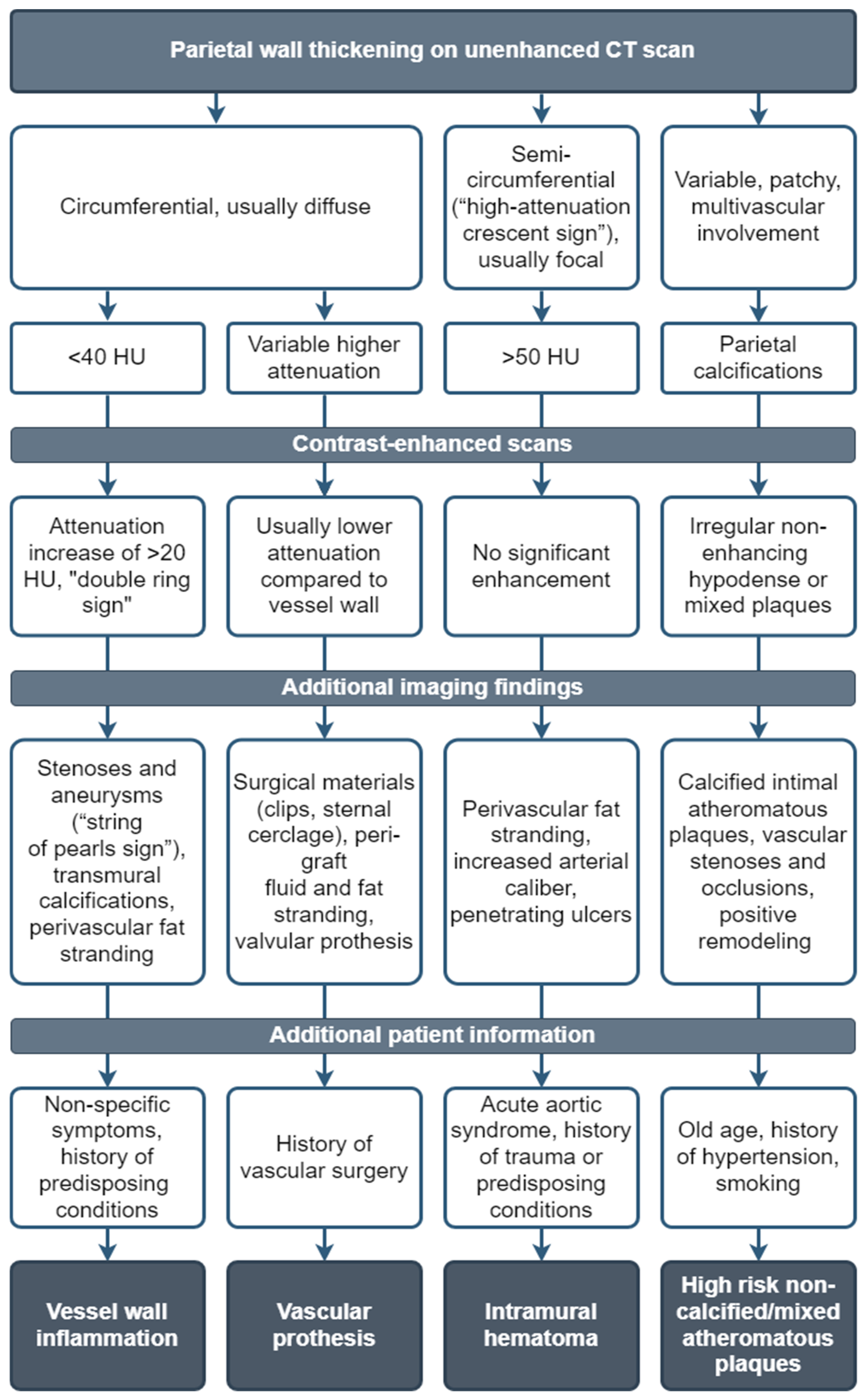
Suggested differential diagnosis workflow approach of the main mimickers of LVV.
Figure 10.
Suggested differential diagnosis workflow approach of the main mimickers of LVV.
Vasculitis nomenclature adopted by the 2012 International Chapel Hill Consensus Conference on the Nomenclature of Vasculitides [4].
Vasculitis nomenclature adopted by the 2012 International Chapel Hill Consensus Conference on the Nomenclature of Vasculitides [4].
| Large vessel vasculitis |
| Takayasu arteritis (TAK) |
| Giant cell arteritis (GCA) |
| Medium vessel vasculitis |
| Polyarteritis nodosa |
| Kawasaki disease |
| Small vessel vasculitis |
| Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis |
| Microscopic polyangiitis |
| Granulomatosis with polyangiitis (Wegener granulomatosis) |
| Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) |
| Immune complex small vessel vasculitis |
| Anti-glomerular basement membrane (anti-GMB) disease |
| Cryoglobulinemic vasculitis |
| IgA vasculitis (Henoch–Schönlein purpura) |
| Hypocomplementemic urticarial vasculitis (anti-C1q vasculitis) |
| Variable vessel vasculitis |
| Behçet’s disease |
| Cogan syndrome |
| Single-organ vasculitis |
| Cutaneous leukocytoclastic angiitis |
| Cutaneous arteritis |
| Primary central nervous system vasculitis |
| Isolated aortitis |
| Others |
| Vasculitis associated with systemic disease |
| Lupus vasculitis |
| Rheumatoid vasculitis |
| Sarcoid vasculitis |
| Others |
| Vasculitis associated with probable etiology |
| Hepatitis C virus-associated cryoglobulinemic vasculitis |
| Hepatitis B virus-associated vasculitis |
| Syphilis-associated aortitis |
| Drug-associated immune complex vasculitis |
| Drug-associated ANCA–associated vasculitis |
| Cancer-associated vasculitis |
| Others |
Main advantages and disadvantages of different imaging modalities in the diagnosis of LVV. Adapted after Schäfer et al. [11] and Dejaco et al. [12].
| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| US | Easily accessible Comfortable for patients Brief acquisition time of around 15 min Suitable for fast-track clinics More cost-effective Very high resolution (up to 0.1 mm in superficial anatomical structures) Strong evidence | Assessment of the thoracic and abdominal aorta is limited Overview of involved vessels is limited |
| CTA | Comprehensive overview of the aorta and its branches Different contrast phases for assessment Clear delineation of atherosclerosis Relatively quick acquisition time | Irradiation of about 17 mSv Contrast agent usage limited by reduced kidney function |
| MRA | Identifies characteristic arterial abnormalities Excellent overview of affected arteries Simultaneous assessment of cranial and extracranial arteries Better visualization of the aorta compared to US No irradiation No iodinated contrast agents | Lower sensitivity compared to CT and US for detecting calcifications More costly than US Claustrophobia Contraindications (cardiac pacemakers or other implanted metal devices) Long acquisition time Limited expertise |
| PET/CT | Good overview of affected arteries Ability to detect differential diagnoses of LVV like infections or malignancies Potentially more sensitive than MRI in detecting disease activity Strong evidence level in LVV | Irradiation of about 25 mSv Costly procedure Not possible in elevated glucose levels Sensitivity significantly decreases after more than 3 days Atherosclerosis, especially in femoral arteries, may be mistaken for LVV |
Imaging findings in primary LVV. Adapted after Aghayev et al. [7].
Imaging findings in primary LVV. Adapted after Aghayev et al. [7].
| Primary Vasculitis | GCA | TAK |
|---|---|---|
| US | “Halo sign” (circumferential hypoechoic rim around the vessel lumen on transversal view)—temporal artery and extremity vessels Positive “compression sign” (no collapse of the inflamed vessel which is still visible after compression) | “Halo sign”—cervical and extremity vessels |
| CTA | Circumferential parietal thickening Vessel wall enhancement | Circumferential parietal thickening Vessel wall enhancement Luminal stenosis or narrowing |
| MRA | Circumferential parietal thickening Vessel wall enhancement Wall enhancement of superficial cranial arteries (on high-resolution MRI) | Circumferential parietal thickening Vessel wall enhancement Luminal stenosis or narrowing |
| PET/CT | Segmental FDG uptake of vessels equal to or more than the liver FDG uptake of shoulder/hip joints or synovium (if associated with Polymyalgia rheumatica) | Segmental FDG uptake of vessels equal to or more than liver |
Source link
Ioana Popescu www.mdpi.com