1. Introduction
Soils are the basis of a functioning food system and source of income for eight billion humans worldwide. Besides providing economic value and nutrition, soil fulfills several other services and ecosystem functions, such as biomass and fiber production, regulation of water, and nutrient cycles. Moreover, soils fulfill an enormous habitat function by hosting 25% of global biodiversity [
1] and are thereby the foundation of the food chains nourishing aboveground species, thus humanity [
2]. In addition, soils are the most important terrestrial C sink, storing 3500–4800 Pg of C [
3], whereas terrestrial vegetation and the atmosphere store only around 800 Pg C each. Therefore, it is of great importance to not just consider soil as an economic resource and means for our global food system but also being a key in climate change mitigation.
Since ancient times, humans have used different forms of organic soil amendments (OSAs) [
4], the most common forms being straw, slurries, manures, and compost. Examples of more modern forms are biogas digestates, sewage sludge biosolids, and biochar. These amendments not just contain nutrients but also varying amounts of C-rich organic compounds. Therefore, the application of OSAs is often used as a way to increase soil organic carbon (SOC) [
5,
6]. However, these different OSAs have different mean residence times (MRTs) in soil and usually (with the exception of biochar) have to be regularly reapplied to maintain the SOC stock increase in the soil.
Biochar is the product of the thermochemical conversion of organic biomass under minimized oxygen supply, also known as pyrolysis. One must distinguish biochar from other carbonaceous material produced from pyrolysis. In contrast to char or charcoal, biochar is specifically produced for the purpose of being applied to soil [
7]. Biochar contains highly aromatic C compounds and only small amounts of N (most of them being polycyclic and not available for plants) and is therefore, in contrast to other common types of OSAs, highly stable against microbial decomposition. Biochar’s MRT is estimated to be 556 ± 483 years as suggested by a recent meta-analysis [
8], which underlines its high SOC sequestration potential. The wide variation in the mean MRT is mainly because of two factors. Firstly, the original studies included in the meta-analysis reported a broad range of MRTs, with estimates up to 891 years. And secondly, because both field and incubation studies were included in the literature assessment. The observation time of incubation studies is shorter than the actual decomposition time of biochar; thus, the MRT must be extrapolated with a high uncertainty and often does not reflect real field conditions. Long-term field observations of biochar stability are therefore urgently needed.
While biochar resides in soil, several reactions with its immediate surrounding environment take place. These processes do not just affect biochar dissipation but lead to physical, chemical, and biological alterations of the biochar particles. Physical alterations include changes in particle size, porosity, and surface area, and chemical alterations affect mostly surface properties [
9]. Surface alterations due to biochar aging are often linked to the sorption of soil organic matter (SOM), leading to increased surface polarity, decreased specific surface area (SSA), and increased surface charge. The process of SOM sorption depends on pH, with a lower pH generally leading to increased SOM sorption [
10]. SOM sorption can, moreover, block pores and thereby prevent microbes and minerals from penetrating into the particle and interacting with the particle’s inside [
11]. Changes in the oxidation state of aged biochar are mostly biologically driven because aging leads to a colonization of soil microbes that oxidize the altered surface [
12]. This then leads to the incorporation of oxygen into surface groups, which makes the surface more hydrophilic, and due to more negative charges, there is a high potential for positive ion retention.
The field aging of biochar leads to multiple processes occurring simultaneously and sequentially. Aging methods and experiments aiming to imitate these processes artificially are less time-intensive than field aging techniques but do not represent the multiple facets of the aging of biochar and their agronomic implications. Field aging experiments are often carried out with a limited duration [
9,
13,
14], and none of them have observed field aging processes on a decadal scale. However, it is critical to understand how aging dynamics impact biochar’s environmental effects and its agronomic benefits on longer time scales. Since aged biochar better reflects what biochar mineralization would look like in hundreds and thousands of years [
12], understanding its process is particularly interesting for long-term SOC sequestration and carbon dioxide removal.
The co-composting of biochar particles before application does not only “load” the biochar with nutrients, thereby making the soil more fertile, but it also mimics the natural aging process of SOM sorption and coating formation. This affects the stability of particles against microbial decomposition, due to the microbial preference to utilize organic substrates on particle surfaces that require less activation energy for metabolization [
9]. Co-composting also enhances the natural oxidation of biochar particles [
11], which as already explained, increases the surface cation exchange capacity (CEC) and thereby nutrient availability for plants, consequentially leading to higher crop yields [
15]. Studies comparing co-composted and pure biochar have found that co-composting increases plant growth but has no negative effect on the long-term stability of biochar [
16,
17]. Whether the advantageous effects of co-composting on soil and biochar properties are limited to only a few years after amendment or if they persist in the long-term is still unknown.
This study aimed to investigate the impact of long-term biochar aging on soil and biochar characteristics after long-term exposure to environmental conditions in a loamy soil under a temperate climate in Bayreuth, Germany. The first objective was to resample the biochar-amended soil after 13 years of aging and create an un-aged reference by mixing unamended control soil with the original biochar, which was sealed for 13 years. The second objective was to analyze a variety of soil and biochar properties such as pH, EC, SOM, soil nutrients, and soil C in order to describe the soil health status as a function of biochar aging. The third objective was to analyze if pristine aged biochar and co-composted aged biochar impact the soil differently in the long-term.
Our working hypotheses are as follows:
2. Material and Methods
2.1. Sampling, Site Characteristics, and Amendment Properties
To test the hypotheses of this study, a long-term biochar field experiment established in 2010 and located at Donndorf, a village close to Bayreuth, Germany, was sampled.
Table 1 summarizes the main characteristics and properties of the experimental site and the used amendments. A more detailed description of its experimental design can be found in Cooper et al. (2020) [
18].
In this experiment, three of the ten treatments from the original Latin rectangle field experiment design (
Figure S1) were selected. The Latin rectangle structure ensures treatment independence, as each treatment appears only once per row and column. The following treatments were applied: pristine biochar at a rate of 31.5 Mg ha
−1, co-composted biochar combined with 70 Mg ha
−1 compost at the same biochar rate, and an untreated control. Each treatment included five field replicates, with the application occurring in May 2010. The commercial biochar (CarbonTerra, Wallerstein, Germany) was made from pine wood chips and produced in a gasification system via slow pyrolysis at 550 °C for 36 h followed by a second step of high-temperature pyrolysis at 800 °C for 2 h. The compost was produced by BKE Bio-Kompost and Disposal/GmbH & Co., Bayreuth, Germany and derived from green litter. The biochar–compost mixture was set up on 17.05.2010 at the “Bindlacher Berg” composting site, before being transported to the field experiment site on 21.05.2010 after four consecutive days of co-composting. The experiment site was under farming cultivation in each of the following years. More details of the farming activities can be found in Cooper et al. (2020) and Gross et al. (2024) [
17,
18].
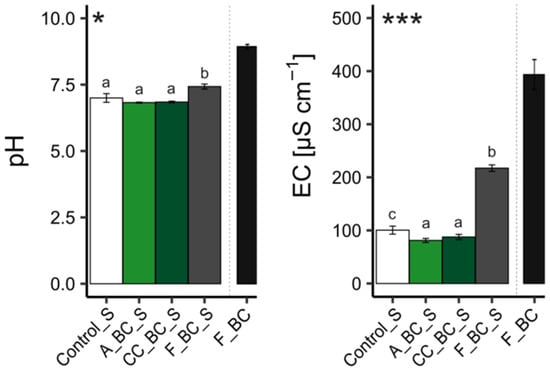
Soil sampling took place in March 2023 before summer sowing. Prior to this, mustard was planted in the fall of 2022 as a cover crop. Samples were collected from five field replicate plots. These plots had been treated with either pristine biochar, co-composted biochar, or left untreated as a control. Biochar amendments were incorporated to a depth of 10 cm using a rotary tiller in 2010. We assumed vertical particle migration with time, and therefore, soil samples in 2023 were taken from a depth of 0–30 cm and combined into composite samples for each treatment group. For clarity, we will refer to the soil treated with aged pristine biochar as “A_BC_S”, the soil treated with aged co-composted biochar as “CC_BC_S”, and the untreated soil as ”Control_S”.
For a better elucidation of the impact of biochar aging on soil properties, we prepared a reference soil by mixing 6 kg of the material from the control site with 472.5 g fresh biochar used as an amendment at the beginning of the field experiment (F_BC_S) in 2010 (
Table 1). This mixture was calculated to replicate the conditions found in the top 30 cm of field soil at the start of the experiment. Given that biochar can undergo aging in the presence of oxygen, the used biochar was stored in sealed plastic buckets to prevent oxidation.
2.2. Soil and Biochar Analysis
The control soil (Control_S) and the biochar-treated soils (F_BC_S, A_BC_S, and CC_BC_S) were analyzed for the following parameters: pH, electrical conductivity (EC), water holding capacity (WHC), soil organic matter (SOM), total carbon (TC), total nitrogen (TN), total phosphorus (TP), soluble phosphorus, available inorganic nitrogen (NH₄+-N and NO₃−-N), specific surface area (SSA), total pore volume, and pore radius. In addition, the biochar (F_BC) used to prepare the fresh biochar-amended soil for this study was also analyzed for pH, EC, and WHC, as well as SSA, total pore volume, and pore radius. It is important to underline that this is the same biochar that was used in 2010 for treating the soil, referred to in this study as A_BC_S. Furthermore, SSA, pore volume, and pore radius were also determined in two additional biochar samples, A_BC and CC_BC; these biochar samples were separated from a small amount of aged biochar-treated soils, A_BC_S and CC_BC_S, respectively.
The pH of the soils and biochar was measured in triplicates (CRISON pH Basic 20) in 1:10 (
w/
v) soil:MiliQ water after being stirred for 30 min and left to rest for another 30 min, following the method described by Campos et al. (2020) [
19]. After pH measurements, the supernatant was filtered, and electrical conductivity (EC) was determined using a conductivity meter (CRISON ECmetro Basic 30) [
20].
The water holding capacity (WHC) of amended and un-amended soils and biochar was determined in 6 replicates by weighing the water retained in 2 g of each material after saturation and subsequent settling for 2 h, in accordance with de la Rosa et al. (2014) [
20]. Maximal WHC was calculated as the ratio of the weight of retained water to the dry weight of the sample expressed in percentage.
SOM content was determined according to the loss-of-ignition method based on the gravimetric weight change associated with the high-temperature oxidation of organic matter. After initial oven drying at 105 °C overnight, the samples were ignited in a muffle furnace for 6 h at 550 °C. The percentage weight loss during the ignition step is the reported SOM (% wt. loss).
Total carbon (TC) and total nitrogen (TN) contents were determined in duplicates by conducting dry combustion using a Flash 2000 elemental micro-analyzer (Thermo Scientific, Bremen, Germany). Total phosphorus (TP) was determined in triplicates following controlled acidic digestion with ultrapure nitric and hydrochloric acid (DigiPREP Block Digestion Systems (SCP Science)) via analysis by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Varian, Santa Clara, CA, USA).
Soluble phosphorus content was obtained in triplicates. Previously dried soil, passed through a 2 mm sieve, was mixed with activated carbon (about 6% w:w) in falcon tubes. Extraction was carried out according to the Olsen method [
21] with a sodium bicarbonate extraction solution at a solid-to-solution mass ratio of 1:20, by shaking for 30 min in a bottle shaker. Supernatants were filtered twice, through folded filters (general filter) and Whatman No. 2 filters in succession, and measured by spectroscopy with a Bran–Luebbe autoanalyzer.
Available inorganic nitrogen (NH₄
+-N and NO₃
−-N) was quantified after the extraction of the samples with 1 M KCl (
w/
w 1:50) for 1 h at 180 rpm, centrifuged for 5 min at 4000 rpm, and filtered through Whatman No. 2 filter paper [
22]. The ionic content was measured in the supernatant by colorimetric assays via Omega SPECTROstar (BMG LABTECH GmbH, Ortenberg, Germany). The NO₃
−-N content of the extract was measured using the salicylic–sulfuric acid method [
23], and NH₄
+-N was determined with an adapted protocol from the colorimetric method described by Greweling and Peech (1960) [
24].
Specific surface area (SSA) and pore volume were determined via adsorption–desorption analysis of N2 at 77 K using an Autosorb iQ Surface Area Analyzer (Quantachrome Instruments, Boynton Beach, FL, USA). Prior to measurement, samples were degassed in a vacuum at 378 K to remove surface adsorbates. SSA was calculated from the adsorption branches using the Brunauer–Emmett–Teller method (BET). The total pore volume was determined by applying the desorption isotherm of the Barrett–Joyner–Halenda (BJH) model. The average pore radius was estimated as a ratio of the total pore volume and SSA.
2.3. Statistical Analyses
Statistical analyses were performed with Microsoft Excel and the software Past 4.03. To determine the significant differences of soil properties due to biochar treatments, a one-way ANOVA was used. Differences were considered statistically significant at
p < 0.05. After finding a significant result in the ANOVA, a post hoc Tukey’s honestly significant difference (HSD) test was employed to compare all possible pairs of means. Pearson’s correlation was used in order to assess the linear relationships between the analyzed parameters (pH, EC, WHC, SOM, TC, TN, NO
3–-N, NH
4+-N, total P, soluble P, SSA, total pore volume, and pore radius). The analysis was performed on three observations; where duplicates were analyzed, the missing values were replaced by mean imputation. For the significance testing,
p < 0.05 was set as the criterion. Principal component analysis (PCA) was conducted on the entire dataset to evaluate the influences of treatments on soil parameter variation. R [
25] was used for visualization.