2.1. Materials and Nanocomposite Preparation
Homopolymer grade poly(vinylidene fluoride) (PVDF) Kynar® 721 (powder form) by Arkema (Philadelphia, PA, USA), with MFR 15 g/10 min (230 °C, 3.8 kg), a melting point of 168 °C, and a glass transition (Tg) of −40 °C was used. Graphene nanoplatelets, with grade SE1233 (GNP), a purity ≥ 97%, a tap density < 0.1 g/cm3, an average diameter D50 ~35–50 μm, and SSA (BET) 400–600 m2/g were supplied by Sixth Element Material Technology Co. Ltd. (Changzhou, China).
2.3. Characterization Methods
Scanning electron microscopy (SEM) was performed to visualize the orientation of layers in the 3D-printed structures. Samples were cut longitudinally in liquid nitrogen and then gold-coated. SEM images of the cross-section were taken at 15 kV accelerating voltage and 110 mA emission current conditions with Tabletop SEM HIROX SH 4000 (Hirox Europe, Limonest, France) at different magnifications.
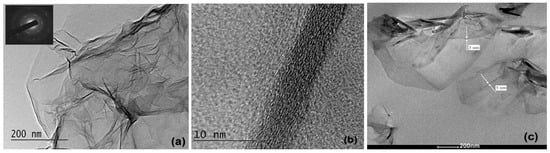
A transmission electron microscopy (TEM) analysis was performed by HR STEM JEOL JEM 2100 (Tokyo, Japan) with high-resolution operation with an acceleration voltage of 200 kV. The preparation of graphene samples was carried out by ultrasonic dispersion of GNP powder in ethanol. Then, a small quantity of the dispersion was placed on standard copper TEM meshes coated with an amorphous carbon membrane and dried at room temperature in a dust-free atmosphere. For the nanocomposite samples, thin sections were cut at room temperature with an ultra-microtome and placed on 400-mesh copper grids. The GNP sheets and the nanocomposite samples were examined at different magnifications to study the hierarchy of their structure from the micro- to nanoscale level.
where ΔHm is the fusion/melting enthalpy (J/g), ω is the actual portion of polymer in the nanocomposite, and ΔHm0 = 104.7 J/g is the melting enthalpy for a 100% crystalline PVDF [32].
A thermo-gravimetric analysis was performed with TGA-Q50 (TA Instruments, USA) by heating from 25 to 800 °C with a ramp-up of 10 °C/min, under nitrogen flow. TG and DTG curves of weight loss and its first derivative were plotted versus temperature. Characteristic temperatures, such as the onset temperature at the start of weight loss (Tonset) and the decomposition peak temperature (Tpeak), were evaluated.
The Joule heating (resistive heating) test was performed by an experimental setup consisting of a power supply (GW Instek, New Taipei City, Taiwan) providing the voltage (V) and measuring the current (I), while the heating temperature was controlled by a thermocouple type K connected to Keithley 6517B (Keithley Instruments, USA). Similarly to the upper test for the resistance measurements, electrical contacts of silver paint were realized on the short sides of the sample and connected to the power supply. The 3D-printed samples with the raster angles of 0°, 45°, and 90° were tested, with the test section of dimension ~20 × 10 mm2 and thicknesses of 0.8 and 2 mm, measured for each sample. Applying voltage to the sample, the local temperature evolution over time was measured by the thermocouple positioned at the center of the sample surface, ensuring close physical proximity to the surface by attaching it with a thermally conductive but electrically insulating LiPOLY AT910 tape (Taoyuan City, Taiwan). The goal was to have thermal coupling for accurate temperature readings without affecting the electrical measurements. Precautions were taken to ensure that no part of the thermocouple came into direct electrical contact with the sample to avoid interference with the current flowing through the sample during heating measurements. Data for the applied voltage and measured temperature, current, and time during four heating and cooling cycles were collected and recorded by the LabView Community edition software. Three samples were tested for each raster angle, and the average values were reported.
Thermo-resistive property characterization was performed, as the resistance was measured by a multimeter Keithley 6517B (Keithley Instruments, USA), while the sample was heated up on a Peltier heating plate of the AR G2 rheometer (TA Instruments, USA) from 25 °C to 160 °C. The controllable temperature was set by the AR G2 (Trios software) with a step of 20 °C and recorded by the thermocouple type K to the Keithley 6517B instrument placed in the middle of the sample. Three samples were tested for each raster angle, and the average values were reported.
where λ is the thermal conductivity [Wm−1 K−1], α is the thermal diffusivity February, Cp is the specific heat [JK−1⋅kg−1], and D is the bulk density [g·cm−3] of the composite. The Proteus® Professional 8.6 software was used for data analysis and calculations of the thermal characteristics.
Source link
Rumiana Kotsilkova www.mdpi.com