2.2. DSC Study of Chelator/DMPC Liposome Mixtures
To investigate the interaction of the chelators with lipid membranes, we used dimyristoylphosphatidylcholine (DMPC) large unilamellar vesicles (LUVs) as a model system due to its high abundance in mammalian membranes and relatively easy preparation protocol [
23]. Chelators stock solutions were prepared in buffer solution (10 mM HEPES, 150 mM NaCl, pH 7.4). The chelator solution was added to the DMPC suspension to achieve a final concentration of 5 mM, and the mixture was incubated for 1 h at 37 °C to allow equilibration. These initial experiments were conducted to obtain insight into the type of interaction of the synthesized chelators with the DMPC liposomes. By testing the chelators at a high concentration of 5 mM, we aimed to identify those that caused detectable changes in the thermotropic properties of the liposome membranes, as observed in the DSC thermograms (
Figure 2).
Table 2 presents the thermodynamic parameters determined based on the heating scans: transition temperature (
Tm), enthalpy change (Δ
H), and the corresponding half-width at half-height (Δ
T1/2) for DMPC in the presence of the chelator in the described conditions.
Literature data indicate that pure DMPC liposomes exhibit a prominent and sharp main endothermic transition around 24 °C, which corresponds to the temperature at which DMPC undergoes a phase transition from the gel (Lβ′) phase to the liquid-crystalline (Lα) phase, also known as the melting temperature,
Tm [
24,
25,
26]. Δ
T1/2 indicates how wide the peak is at half of its maximum height and is a measure of the cooperativity of the phase transition. For pure lipids of DMPC, the pre-transition usually either cannot be observed for the LUVs, or it appears as a “shoulder” superimposed on the main phase transition curve.
The DSC data (
Table 2) show that increasing the alkyl chain length, starting from butyl-derivatives, decreases the melting temperature (
Tm) of DMPC liposomes. This suggests that longer alkyl chains destabilize the membrane structure, reducing the temperature required for the gel-to-liquid-crystalline phase transition.
Interestingly, the enthalpy and cooperativity parameters remained mostly unchanged, indicating that the overall energy of the phase transition and the sharpness of this transition were not significantly affected. Only a slight increase in enthalpy was observed for hexylmpp. However, although the ΔH values reported here provide a general trend, they should be interpreted with caution. It is not possible to definitively conclude whether subtle changes in ΔH have occurred, as the experimental method may not have the necessary precision to detect them.
The constant enthalpy suggests that the bilayer’s structural integrity is maintained, but the organization of the lipid tails is influenced by hydrophobic interactions between the alkyl chains and the lipid tails. The progressive decrease in
Tm with longer chains could be linked to an increase in the disorder of the lipid tails, as the larger hydrophobic moieties disturb the packing of DMPC, weakening the van der Waals interactions between lipid molecules. In fact, the compounds can interact with lipids in biomembranes intercalating between the flexible acyl chains of lipids causing
Tm variations but no Δ
H changes [
27].
For DMPC/hexylmpp and DMPC/hexyletpp mixtures, a marked reduction in Tm was observed compared to the other derivatives. The main peak moves from Tm = 24.4 °C of pure DMPC to 22.5 °C and 21.5 °C, respectively, suggesting that the longer hexyl chain has a pronounced effect on the lipid bilayer structure.
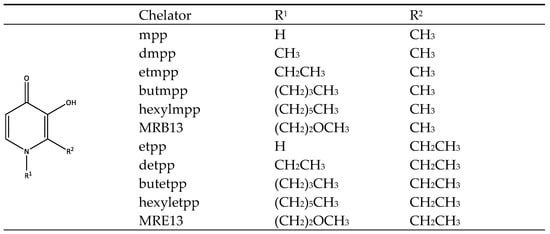
The overall data also reveals that the changes in the thermodynamic parameters were solely influenced by the different R1 substituents on the pyridine ring, while the R2 substituents had little to no effect on the studied parameters. Therefore, it can be concluded that both groups of ligands tested, derived from the two pyrones (with R2 = CH3 or CH2CH3, respectively), exhibit similar interactions with DMPC liposomes, with these interactions being dependent on the substituents on the nitrogen atom of the heterocyclic ring.
Subsequently, the chelators that influenced the thermotropic behavior of DMPC suspensions were further investigated to assess their effects at variable concentrations (0.5 to 10 mM). This allowed us to better understand the concentration-dependent impact of each chelator on the lipid bilayer, providing insights into the strength and nature of the chelator-membrane interaction.
Figure 2 shows the DSC heating plots for selected DMPC/chelator mixtures. The thermodynamic parameters are depicted in
Table 3.
Hexyl derivatives induced a gradual decrease in the main transition temperature (Tm) with increasing concentration, while cooperativity and enthalpy remained almost unchanged, for the tested concentrations, which is consistent with the findings observed at 5 mM. This suggests that the hexyl group increases membrane fluidity without significantly altering the overall organization or energy of the phase transition, despite the lowered Tm.
In contrast, butyl derivatives exhibited a much smaller effect on Tm, which may suggest that the shorter alkyl chain has a less pronounced impact on the lipid bilayer’s phase transition. The minimal effect of the butyl group might be related to its lower hydrophobicity or less significant disturbance of the lipid packing compared to the longer hexyl chain.
Chelators like MRB13 and MRE13 had no effect on thermodynamic parameters, possibly due to their higher hydrophilicity given by the presence of polar atoms, particularly oxygen [
13], which limits interaction with the hydrophobic liposome bilayer.
2.3. EPR Study of Chelator/POPC and Chelator/DMPC Liposome Mixtures
For the EPR studies, POPC and DMPC were used. Similarly to DMPC, POPC is extensively used as a mimic for eukaryotic membranes and, thus, it can represent the human cell membrane, being, in fact, the most common phospho-lipid type [
28]. POPC is a more fluid bilayer at room temperature (
Tm = −2 °C) due to the unsaturation in its acyl chain. DMPC has a well-defined phase transition temperature (around 24 °C), being in a gel-solid state below it and fluid above. Selecting 37 °C for DMPC ensures it is in the fluid phase, simulating physiological conditions and enabling comparisons between the models.
EPR studies with POPC and DMPC liposomes labeled with 5-DSA and 16-DSA spin probes provide additional insights into how the chelators modulate membrane fluidity (see
Figure 3) and allow the determination of the site of the interaction within the membrane. The results indicate that etmpp and butmpp alter the membrane fluidity of both liposomes, primarily affecting the region near the polar head groups (as detected by the 5-DSA probe). On the other hand, hexylmpp only influences the head group region of DMPC (
Figure 3a,c).
Interestingly, the 16-DSA probe, located deeper within the lipid bilayer, exhibits distinct behavior in the two liposome types. While different compounds (etmpp, butmpp, hexylmpp, and hexyletpp) induce changes in POPC, only hexylmpp significantly impacts DMPC (
Figure 3b,d). The selective modifications detected by the 5-DSA and 16-DSA probes highlight the role of both the liposome composition and the structural properties of the compounds. In DMPC and POPC liposomes, the effects detected by the 5-DSA probe suggest that etmpp and butmpp interact predominantly near the polar head group region, which is consistent with their moderate hydrophobicity and likely localization closer to the bilayer interface. The unique response of the 5-DSA probe to hexylmpp in DMPC implies that this compound may insert into the bilayer differently, possibly due to its longer alkyl chain, which enhances its partitioning into the lipid bilayer while maintaining interaction with the polar head group region in this liposome.
The 16-DSA probe results, which monitor deeper regions of the bilayer, show that in DMPC only hexylmpp induces detectable changes, suggesting that this compound has sufficient hydrophobic character to penetrate deeper into the bilayer core. This observation aligns with the tight packing and lower fluidity of DMPC bilayers, which may restrict the deeper insertion of shorter-chain compounds like etmpp and butmpp.
In contrast, the effects observed in POPC liposomes differ significantly. The 16-DSA probe shows changes in fluidity with etmpp, butmpp, hexylmpp, and hexyletpp, indicating that these compounds can penetrate deeper into the bilayer in this more fluid and unsaturated membrane system. This enhanced accessibility to the hydrophobic core in POPC compared to DMPC may result from the intrinsic differences in lipid packing and fluidity due to the unsaturated acyl chains in POPC. The fact that hexylmpp increases fluidity only in the 16-DSA region of POPC further supports its integration into the hydrophobic core without strongly affecting the more surface-exposed lipid regions.
Interestingly, the 2-ethyl derivatives, (
Figure 1) show no interaction, a result that is not fully understood. This effect might result from weak or transient interactions between the derivatives and the membrane, enough to alter phase transition properties but insufficient to induce notable fluidity changes at the molecular level or could promote interactions at the membrane-water interface, influencing the overall packing and phase behavior without substantially affecting the bilayer’s dynamic properties probed by EPR. In fact, the presence of the ethylene group on the R
2 position of the pyridinone ring, usually has an impact in the hydrophilicity of the ligands, as well as their respective metal ion complexes. We suppose that these ligands are probably less soluble in an aqueous medium, thus forming microscopic precipitates and, therefore, compromising the interaction of these molecules with liposome membranes. For example, a study considering iron(III) complexes derived from maltol (Fe(mpp)
3 and Fe(dmpp)
3) or from ethylmaltol precursors (Fe(etpp)
3), showed that the methyl derivatives, (mpp and dmpp), have a superior effect on the fertilization of soybean plants, while the ethyl derivative (etpp) demonstrated a less pronounced effect [
29]. Once again, biophysical studies pointed out that these differences were due to the lower hydrophilicity of the etpp-derived complex in comparison with the maltol-derived ones.