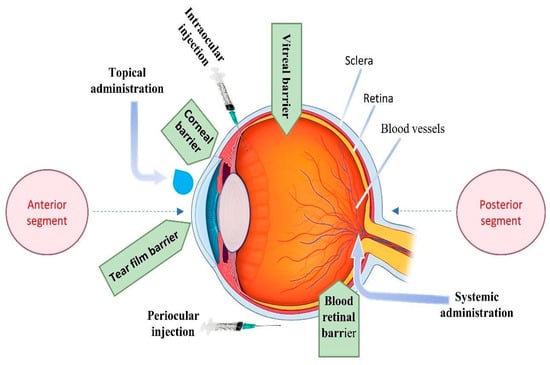
Recent advancements have highlighted the important function of LLCs in improving the delivery of medication through mucosal surfaces. These systems are highly appreciated for their exceptional capacity to efficiently penetrate biological barriers and sustain a long-term presence at the site of administration, which has the potential to enhance therapeutic effectiveness [66]. LLCs are created through the manipulation of the concentration of specific substances, such as surfactants, within a specific range of temperatures. As the solute concentration increases, distinct mesophases become apparent, indicating varied structural and functional properties [67]. The mesophases, namely the lamellar, hexagonal, and cubic phases shown in Figure 4, result from the self-organization of amphiphilic lipids due to variations in temperature and composition.
The self-assembly process is primarily driven by hydrophobic interactions that take place when these lipids are exposed to water, resulting in the formation of organized aggregates [68]. The reverse cubic and reverse hexagonal phases are highly suitable as delivery systems for a wide range of medicinal applications among the LLCs [69]. The internal composition of these stages enables the inclusion and secure containment of different therapeutic molecules, including those that are water-loving, fat-loving, and both water-loving and fat-loving in nature [70]. This provides possibilities for precise and regulated release mechanisms [71]. The cubic phase LC structures display a distinct duality in their structural formation. They can either appear as typical micellar aggregates with continuous water channels and fragmented hydrocarbon regions or as reversed aggregates with the opposite configuration [72]. Their ability to adapt structurally contributes to their ability to control the release of drugs. In addition, cubic phase LLCs exhibit notable mechanical rigidity in comparison to lamellar and hexagonal LCs, thereby impeding the unregulated movement observed in these mesophases [73]. When there is too much water added, the cubic phase changes into a clear gel that is thick and stretchy [74]. This gel is stable and has the same properties in all directions. These gels, although stiff, are difficult to detect using conventional polarized electron microscopy, emphasizing their distinctive physical properties [60]. The hexagonal phase is distinguished by the arrangement of cylindrical micelles into a three-dimensional hexagonal lattice. When exposed to organic solvents, this structure can undergo reversal, resulting in the formation of the reverse hexagonal phase. When adequately mixed with water, these hexagonal structures exhibit a unique fan-like texture that is visible when observed under polarized light microscopy [58]. Ultimately, the lamellar LCs, also known as neat phases, expand significantly, creating substantial structures resembling sheets that are interspersed with channels of water. The presence of a bilayer structure in these phases reduces the interaction between the oil and aqueous phases, which is advantageous for the encapsulation and preservation of drugs. The arrangement can be observed using polarized light microscopy, which reveals a unique streaky or mosaic-like texture, indicating its anisotropic characteristics [60].
LLCs for ocular drug delivery are categorized into two main types based on their formulation strategy, bulk-forming liquid crystals and nanoparticle liquid crystals (LCNPs). Bulk-forming liquid crystals are extensively studied for their capacity to create sustained release systems. These bulk phases, including hexagonal and cubic structures, are engineered to maintain therapeutic levels of drugs over extended durations [78], whether applied locally or intravitreally, thereby enhancing the treatment efficacy for various ocular conditions. Conversely, liquid crystal-based nanoparticles, such as cubosomes, represent a significant advancement in ocular drug delivery by improving drug targeting, controlled release, and bioavailability [79]. Formulated with amphiphilic lipids, these nanoparticles effectively deliver therapeutic agents to the eye, overcoming traditional barriers and ensuring sustained therapeutic effects. Both bulk-forming and LCNPs exemplify innovative approaches in ocular drug delivery, offering substantial improvements in treatment outcomes for a range of eye diseases.
5.1. Bulk-Forming Liquid Crystals for Ocular Drug Delivery
Glycerol monooleate (GMO) is currently a highly researched amphiphilic lipid due to its potential use in the development of LLC medication formulations [80]. GMO, which is included in the FDA Inactive Ingredients Guide, is known for its non-toxic, biodegradable, and biocompatible properties. It is classified as Generally Recognized as Safe (GRAS) [81]. This approval highlights the potential appropriateness of using it in pharmaceutical applications, specifically for delivering therapeutics like vancomycin, as investigated by Milak et al. (2019) [68]. Milak et al. conducted extensive research on the utilization of GMO to create bulk hexagonal and cubic phases that are specifically tailored for ocular drug delivery systems [68]. These systems are designed to maintain therapeutic levels of Vancomycin HCl either locally in the eye or intravitreally for long periods of time. Their innovative method utilized melted homogenization and solvent evaporation techniques to create these phases, which effectively regulated the release rate of Vancomycin HCl [68]. The hexagonal phase, characterized by closed water channels, was found to considerably delay the release of Vancomycin HCl in comparison to the reverse cubic phase, which has open water channels [68]. Milak et al. conducted additional research in 2020 to examine the structural and release properties of liquid crystalline phases made from GMO/Paraffin oil/Vancomycin HCl. The liquid crystalline phases were altered by incorporating additives like polyglycerol ester or a triblock copolymer to assess and improve their capacity to sustain therapeutic Vancomycin HCl concentrations over prolonged durations [82], whether applied topically or injected intravitreally.
The objective of the study was to enhance the compositions for long-term treatment of eye infections. The results demonstrated encouraging prospects for both localized and systemic ocular applications [82].
Phytantriol (PHY) is another notable example of amphiphilic lipids that has been recognized for its non-toxic, mucoadhesive, and biocompatible characteristics. It is commonly used as a secure and efficient framework in LLC formulations [83]. Wang et al. (2019) utilized PHY to create a reversed bicontinuous cubic phase carrier for the transportation of pilocarpine nitrate, a medication frequently employed in the treatment of glaucoma [83]. The team aimed to reduce ocular irritancy and improve the bioavailability of pilocarpine nitrate by using a vortex method to mix PHY and water in a specific ratio. The LC gels produced were evaluated against conventional eye drops and exhibited a sustained release pattern, reduced corneal irritation, and prolonged drug retention in the ocular environment. These findings indicate a substantial enhancement over conventional delivery techniques [83]. Building upon this study, Xingqi et al. further examined the capability of PHY-based LC gels containing cubic and hexagonal phases for delivering pilocarpine nitrate to the eye [71]. Their research showed that these gels can effectively keep therapeutic levels of pilocarpine nitrate in the liquid part of the eye for at least 12 h after being given, which significantly improves the drug’s ability to be absorbed by the body and offers a more efficient treatment for glaucoma [71]. Additionally, Wu et al. created a novel in situ LC gel specifically formulated for the ophthalmic administration of dexamethasone [84]. This system was specifically designed to improve the retention of dexamethasone in the eye, resulting in higher levels of dexamethasone available in the eye and proving to be especially effective in treating DR [84]. Their research confirmed that the in situ-formed liquid crystal gel (ISLG) can release substances over an extended period, is highly compatible with living organisms, and is safe. This positions the ISLG as a promising foundation for future treatment approaches in managing ocular diseases [84]. Li et al. developed a new type of gel called resveratrol-loaded ocular lamellar crystalline gel (ROLG) to treat corneal neovascularization [66]. The research team employed advanced imaging techniques, such as polarized light microscopy and small-angle X-ray scattering, to confirm the presence of lamellar crystalline structures in the ROLGs. These gels exhibited strong retention properties on the surface of the eye, outstanding capacity to load drugs, and improved drug permeability through the tissues of the cornea. In addition, the gels were recognized for their simplicity of application and ability to provide a sustained release, making them an optimal vehicle for delivering resveratrol and a potentially effective new treatment option for managing corneal neovascularization [66]. Also, Tarsitano et al. investigated the use of LLCs as a novel ocular drug delivery system for the delivery of Acyclovir [85]. The study successfully demonstrated the use of a lamellar phase LC that transitions to a cubic phase in situ on the corneal surface, allowing for controlled drug release while overcoming issues with the physicochemical properties and limited precorneal retention time of many ophthalmic medications. The cubic phase exhibited constant viscosity and degradation kinetics across a variety of circumstances, indicating a significant potential for improving drug delivery efficacy. Ex vivo investigations on pig eyeballs and isolated corneas further proved the system’s stability and safety, with no deleterious alterations identified in corneal tissue structures [85]. The wide range of research highlights the powerful ability of LLCs to transform ocular drug delivery. Table 4 provides a summary of bulk-forming LLCs utilized in ocular drug delivery.
5.2. Liquid Crystal-Based Drug Nanoparticles (LCNPs) for Ocular Drug Delivery
The LCNPs are nanoscale particles that combine the ordered structure of crystals with the fluidity of liquids [86]. Formed by the self-assembly of amphiphilic molecules in solvents, LCNPs can encapsulate both hydrophilic and hydrophobic drugs, offering controlled release and enhanced bioavailability for drug delivery applications [86].
The application of LCNPs in ocular drug delivery has made remarkable progress, demonstrating improvements in therapeutic targeting, controlled and sustained release, stability, bioavailability, and the solubility of poorly soluble drugs. Research in this field over the past decade has established a strong foundation for ongoing and future innovations, confirming LCNPs as a transformative approach in ocular pharmacotherapy.
In the presence of water, lamellar phase LC structures commonly produce liposomes, which are spherical vesicles made up of one or more lipid bilayers. On the other hand, cubic and hexagonal phases can retain their unique geometric structures, resulting in the formation of cubosomes and hexosomes, which are nanoparticles with a well-defined internal architecture that is suitable for encapsulating and releasing drugs [67]. A pioneering study by Gan, Li et al. marked the inception of self-assembled LCNPs (cubosomes) for ocular dexamethasone delivery, demonstrating significantly enhanced aqueous humor pharmacokinetics compared to conventional eye drops. The study revealed that low-viscosity cubosomes containing 10% oil remained in the preocular region longer, leading to an eight-fold increase in the area under the curve (AUC) for dexamethasone compared to standard formulations [35].
Building on this foundation, Chen et al. (2012) developed LCNPs for cyclosporine A delivery, utilizing a combination of GMO and poloxamer 407 [81]. This formulation markedly improved corneal penetration and retention, surpassing the performance of oil solutions and offering a promising approach for treating ocular conditions with reduced irritation.
Similarly, Li et al. (2013) investigated LCNPs for pilocarpine nitrate delivery in glaucoma treatment. Their formulation, characterized by uniformly dispersed nano-sized particles, exhibited minimal ocular irritation, enhanced bioavailability, and sustained intraocular pressure reduction, presenting a significant advancement over commercial eye drops [87].
Further refining the application of LCNPs, Achouri et al. (2015) employed a design of experiment methodology to optimize nanoparticle formulations for keratoconus treatment. Their research identified critical parameters such as temperature, emulsification length, and homogenization, which are essential for achieving optimal particle size and drug encapsulation efficiency [88].
Hartnett et al. (2015) highlighted the stability of cubosomes derived from amphiphiles like PHY and GMO, which are well suited for prolonged drug release [89]. This stability is crucial for maintaining consistent therapeutic levels in treatments that require sustained medication release.
In 2016, Liu et al. introduced an innovative ocular delivery method using tetrandrine-loaded LCNPs [90]. This formulation demonstrated significantly improved ocular bioavailability and pharmacological benefits, suggesting a novel approach for enhancing drug retention and efficacy in eye treatments. Concurrently, Verma and Ahuja (2016) evaluated cubic LCNPs for tropicamide delivery, finding that these nanoparticles provided a faster onset of action and increased potency, offering potential improvements in eye care procedures [91].
Ali et al. (2016) developed a cubosomal system for ketorolac delivery, which improved transcorneal permeability and retention, highlighting the potential of cubosomes as a more effective alternative to traditional eye drops [92]. Younes et al. (2018) further advanced this field by creating a cubosomal delivery system for sertaconazole nitrate, enhancing the drug’s permeability and effectiveness in treating fungal keratitis—a significant challenge in ocular pharmacotherapy [93].
Recent studies have continued to push the boundaries of LCNP applications. Silva et al. (2019) designed a pirfenidone-loaded LCNP system that accelerated corneal healing, demonstrating the potential of LCNPs in treating corneal injuries [94]. Eldeeb et al. (2019) explored brimonidine tartarate-loaded cubosomes, which significantly improved the efficacy and permeability of the drug, offering a more effective and long-lasting treatment for glaucoma [95]. The promise of cubosomes in the treatment of glaucoma was highlighted by the findings of the study, which showed a 4.6-fold increase in AUC and a 1.6-fold enhancement in permeability in comparison to any commercially available products [95].
El-Gendy et al. (2020) investigated the inclusion of penetration enhancers in cubosome formulations, finding that these customized liquid crystalline nanostructures enhanced ocular drug delivery without causing irritation, thereby expanding their potential applications [96]. Kaul et al. (2021, 2022) studied the use of LCNPs for delivering tobramycin and vancomycin, showing improvements in preocular residence time and drug permeability, which could reduce dosing frequency and enhance treatment efficacy [97,98].
In 2021, Bessone et al. advocated for the use of cubic LCs for latanoprost delivery, demonstrating their ability to release the drug continuously over an extended period, potentially revolutionizing glaucoma treatment by reducing dosing frequency and limiting adverse effects [99]. Elfaky et al. (2021) developed a ketoconazole cubosomal gel that enhanced drug penetration and retention while displaying strong antifungal activity, highlighting the therapeutic potential of cubosomal formulations [100].
Recent advancements include Shoman et al.’s (2023) exploration of hyaluronan-based cubosomes loaded with bromfenac sodium, which improved corneal permeability and retention, potentially enhancing treatment outcomes for pterygium and cataract [101]. Nasr et al. (2023) revealed that fluconazole combined with cubosomal nanoparticles exhibited superior penetration and safety profiles, offering a more efficient topical therapy for keratomycosis [102]. According to the findings of both the ex vivo and in vivo trials, the corneal penetration was significantly higher, and the therapeutic effects were superior to those of aqueous solutions [102].
Further innovations in 2023 by El-Gendy et al. focused on improving the ocular bioavailability and therapeutic efficacy of Travoprost using liquid crystalline nanostructures [103]. Priya et al. (2023) developed a Loteprednol Etabonate-loaded LCNP gel, which demonstrated improved ocular retention and efficacy, marking a significant advancement in treating inflammatory eye conditions [104]. Similarly, Malaekeh-Nikouei et al. (2023) produced fluorometholone-loaded cubosomes, providing insights into efficient steroid delivery for ocular inflammation [105]. The formulation that was optimized did not exhibit any major changes in terms of physical characterization or in vitro release, which is evidence that the formulation showed perfect stability.
In a study by Sharadha et al. (2023), triamcinolone-loaded cubic LCNPs outperformed typical suspensions in drug delivery and therapeutic outcomes, suggesting the potential for improved nanocarriers for retinal therapeutics [106]. Omran et al. (2024) introduced a phytocubosomal system coated with chitosan and loaded with luteolin, demonstrating significant success in reducing intraocular pressure and inflammation in glaucoma treatment [107]. Those phytocubosomes exhibited sustained drug release, enhanced antioxidant activity, and increased ex vivo transcorneal penetration in comparison to the luteolin suspension.
Iyer et al. (2024) investigated an extended release cubogel formulation of moxifloxacin hydrochloride, showing potential for continuous release and enhanced bioavailability, positioning it as a promising alternative to traditional eye drops [108]. Chakorkar et al. (2024) optimized fluorometholone-loaded cubosomal vesicles using a quality by design approach, demonstrating improved drug release and ocular bioavailability [109]. Aher et al. (2024) examined acetazolamide-loaded cubosomes, highlighting enhanced corneal penetration and prolonged drug release, which could improve glaucoma treatment efficacy and patient compliance [110].
Finally, Nemr and Adel (2024) developed a fenticonazole-loaded cubosomal formulation that significantly improved corneal absorption and permeation, offering a transformative approach to treating fungal eye infections [111]. Bhageerathy and Prasanth (2024) furthered this research by demonstrating that a prolonged release cubogel formulation of moxifloxacin hydrochloride could provide sustained drug release and increased bioavailability, potentially improving bacterial conjunctivitis therapy [112].
The enormous body of research that has been conducted over the course of more than a decade highlights the revolutionary potential of drug nanoparticles based on LCs in the context of ocular drug delivery. The aforementioned developments shed light on the capability of licensed clinical practitioners (LCNPs) to boost patient compliance, reduce the frequency of doses, and improve therapeutic outcomes, thereby paving the way for future innovations in ocular pharmacotherapy. In order to fully grasp the benefits of these advanced delivery methods in treating a wide variety of ocular disorders, it will be necessary to conduct additional research and clinical validation as the area continues to undergo development. Table 5 provides a summary of LCNPs utilized in ocular drug delivery.
While traditional LLC applications have focused predominantly on their structural versatility and controlled drug release properties, recent advancements have significantly broadened the scope of their utility.
Source link
Samer Adwan www.mdpi.com