Doxorubicin (DOX) is a broad-spectrum antitumor drug widely used in clinical practice. Its clinical application is extensive and holds significant importance, particularly in the following domains. DOX emerges as a cornerstone medication in the therapeutic repertoire for acute leukemia, exhibiting remarkable efficacy in addressing both acute lymphoblastic leukemia and acute myelogenous (granulocytic) leukemia [1]. Often serving as the foundation of treatment protocols, it becomes an invaluable second-line therapy when initial drug choices demonstrate resistance [2], thereby expanding the array of potential therapeutic interventions. DOX also demonstrates exceptional efficacy in the management of both non-Hodgkin’s lymphoma and Hodgkin’s lymphoma, functioning either as the primary therapeutic agent or as a critical component within a multidrug regimen [3,4]. Its potent antitumor activity underscores its importance in the treatment paradigm of malignant lymphomas. Breast cancer represents another key therapeutic indication for DOX [5]. In its treatment strategy, DOX is frequently administered in combination with drugs such as cyclophosphamide, paclitaxel, or other DOX-based regimens to enhance therapeutic efficacy [6,7]. This synergistic combination has become a fundamental aspect of the standard of care for breast cancer treatment. Beyond these malignancies, DOX boasts a versatile application in the treatment of bladder cancer, head and neck cancers, testicular cancer, liver cancer, stomach cancer, and numerous other tumor types [8,9,10,11,12,13,14]. Its broad-spectrum antitumor properties emphasize its pivotal role in the therapeutic repertoire against a diverse array of cancers.
Why does DOX occupy such a pivotal role in clinical practice? Firstly, its remarkable efficacy spans a wide range of tumor types, rendering it invaluable in clinical settings due to its broad-spectrum antitumor capabilities. Its therapeutic prowess is evident in both benign and malignant tumors, underscoring its versatility and significance in clinical applications. Secondly, its potent antitumor activity is a key factor contributing to its effectiveness. By interfering with the chemical structure of DNA and inhibiting nucleic acid synthesis, DOX eliminates tumor cells at a deeper level [15,16]. This potent antitumor activity enables DOX to excel in the treatment of a diverse array of refractory tumors. Furthermore, DOX is highly favored as a component in combination therapy regimens. It holds an indispensable position in numerous combination chemotherapy protocols, where its synergistic interaction with other anticancer agents significantly enhances therapeutic efficacy and mitigates drug resistance [17,18]. Consequently, this contributes to the prolongation of patient survival. In conclusion, as a broad-spectrum antitumor agent, DOX embodies immense value and significance in clinical practice. Its extensive applicability and outstanding efficacy have served as a beacon of hope and provided relief to numerous tumor patients.
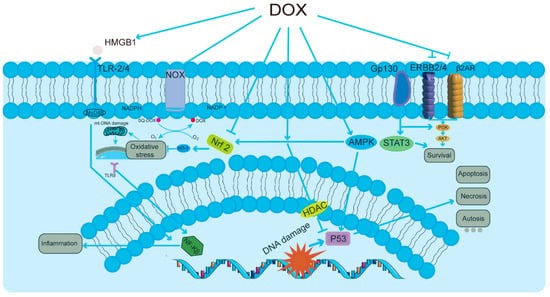
Nonetheless, a major challenge in the clinical application of DOX lies in its dose-dependent cardiotoxicity, posing a formidable limitation on its utilization [19,20,21]. One of the primary characteristics of doxorubicin-induced cardiotoxicity (DIC) (Figure 1) is the initiation of cellular responses in cardiomyocytes [22,23,24], which may lead to congestive heart failure. Among these pathways, the activation of the HMGB1/TLRs/NF-κB signaling pathway leads to heightened inflammatory responses. In parallel, the NOX/Nrf2/HO-1/ROS pathway plays a significant role in generating reactive oxygen species, thereby contributing to oxidative stress. Additionally, the AMPK/P53 pathway is responsible for inducing cell cycle arrest and apoptosis, while the PI3K/AKT/STAT3 pathway facilitates survival signaling. Together, these interrelated processes result in oxidative stress, mitochondrial DNA dysfunction, increased inflammation, and various forms of cell death, including apoptosis, necrosis, and autophagic cell death (autosis), ultimately affecting tissue homeostasis and function, as shown in Figure 1. This adverse effect not only diminishes the quality of life for cancer patients but also significantly increases the risk of mortality. Among these deleterious effects, mitochondrial damage stands out as particularly pronounced [25,26]. DOX first accumulates in the mitochondria of cardiomyocytes, where it binds to the phospholipid cardiolipin in the inner mitochondrial membrane, blocking the electron transport chain and inhibiting the function of complexes I and II, leading to a significant increase in reactive oxygen species (ROS). Excessive production of ROS disrupts the mitochondrial membrane lipids, which further leads to mitochondrial dysfunction and cell death [27,28]. DOX binds to DNA and topoisomerase 2β (Top2β) to form the Top2–DOX–DNA cleavage complex, which triggers cell death [29]. The inhibition of Top2β, an enzyme specific to cardiac mitochondria, leads to further disruption of mitochondrial structure and function [30]. Moreover, a novel type of cell death, “iron death” (Ferroptosis), is also involved in DIC [31]. DOX upregulates heme oxygenase 1, releasing free iron and forming an active iron–DOX complex, which further promotes the oxidation of mitochondrial membrane lipids, leading to cell death [32]. Autophagy is an important mechanism by which cells remove damaged mitochondria and maintain homeostasis [33]. DOX affects the PI3Kγ pathway and activates the PI3Kγ/Akt/mTOR cascade by inhibiting autophagic flux and suppressing the autophagy initiation process, thus further exacerbating the impairment of mitochondrial function [34]. DOX-mediated ROS production and lipid peroxidation can alter the activity of membrane proteins, such as mitochondrial calcium channels, thereby affecting Ca2+ homeostasis and signaling [35,36]. In addition, DOX impairs the expression and activity of myocardial sarcoplasmic reticulum ryanodine receptor 2 (RyR2) and sarcoplasmic reticulum Ca2+-ATPase (SERCA2), major participants in myocardial contraction, further reducing myocardial contractility [37,38]. In conclusion, the primary features of DOX’s dose-dependent cardiotoxicity include the impairment of cardiac mitochondrial function, which involves several facets of ROS production, the inhibition of Top2β, induction of iron death, the inhibition of autophagic flux, and the disturbance of Ca2+ homeostasis. Together, these interrelated pathways cause damage to cardiomyocytes and a reduction in heart function.
However, there is no systematic overview of the various modes of action of the mitophagy mechanism in DIC so far, nor is there a comprehensive assessment of the drugs and potential molecular targets that can be used to treat DIC with therapeutic efficacy from the perspective of mitophagy. Therefore, studies exploring the role of mitophagy in DIC are important for understanding its pathogenesis and developing effective treatments.
Source link
Heng Zhang www.mdpi.com