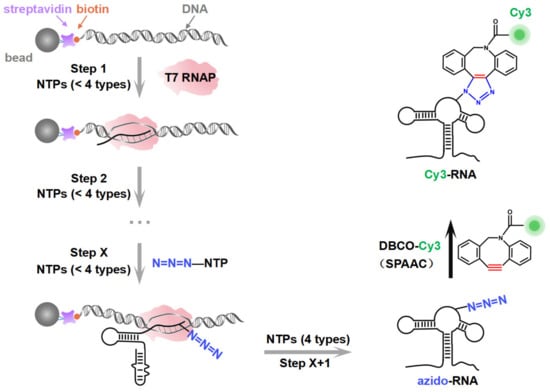
2.1. Incorporation of Cy3 Fluorophore into Specific Sites of riboSAM Using ePLOR
Taking riboSAM as the target for labeling and synthesis, we first introduced an azide group into the U43 position within riboSAM’s loop2 using a seven-step PLOR reaction to obtain U43 azido-riboSAM (Figure 3A). All of the RNA and DNA sequences used in the PLOR are shown in Table S1, and diagrams of the PLOR generation of U43 azido-riboSAM are shown in Figure S2 and Table S2. Subsequently, we investigated the SPAAC reaction system between U43 azido-riboSAM and DBCO-Cy3. Given the DBCO content-dependent enhancement of reaction efficiency in SPAAC [22], we set up experiments with DBCO-Cy3 in molar amounts that were 1, 10, 50, 100, 250, 500, 750, and 1000 times that of U43 azido-riboSAM, and we used urea-PAGE to detect the production of U43 Cy3-riboSAM. Fluorescent gel imaging indicated that little U43 Cy3-riboSAM was generated in the presence of DBCO-Cy3 when the concentration of DBCO-Cy3 was below 50 times (i.e., 1 or 10 times). As the DBCO-Cy3 concentration further increased, the production of U43 Cy3-riboSAM gradually elevated, tending to reach the maximum reaction yield at 500 times and above (Figure 3B, upper part and Figure 3C). UV gel imaging displayed the same results (Figure 3B, lower part). Specifically, on the UV gel, U43 Cy3-riboSAM exhibited a shifted band in comparison to U43 azido-riboSAM due to the incorporation of the Cy3 group, which caused the U43 Cy3-riboSAM to migrate slightly slower. Furthermore, when the DBCO-Cy3 concentration reached 500 times or higher, virtually all of the U43 azido-riboSAM was converted to U43 Cy3-riboSAM. This demonstrated that the SPAAC reaction on RNA achieved an almost perfect reaction efficiency of approximately 100% (Figure 3B, lower part). After removing the excess DBCO-Cy3 through concentration and solvent exchange, we also used reverse-phase high-performance liquid chromatography (RP-HPLC) to characterize the U43 Cy3-riboSAM generated under a condition with a 1000-fold molar excess of DBCO-Cy3. In RP-HPLC, U43 Cy3-riboSAM eluted from the C8 column later than U43 azido-riboSAM (Figure S3), and was detectable upon fluorescence excitation at 550 nm. These observations were attributed to the hydrophobic and fluorescent properties of Cy3, further confirming the successful incorporation of Cy3 into the riboSAM. Moreover, under UV irradiation, the HPLC chromatogram revealed a single predominant peak corresponding to U43 Cy3-riboSAM, accompanied by only a minimal, nearly imperceptible peak attributable to U43 azido-riboSAM (Figure S3B). This observation strongly suggests that the SPAAC reaction proceeded with an efficiency approaching 100%, effectively converting virtually all of the U43 azido-riboSAM into U43 Cy3-riboSAM.
Under the conditions of the DBCO-Cy3 concentration being 500 times that of U43 azido-riboSAM, we further investigated the reaction rate. Urea-PAGE detection showed that the reaction between the two was extremely rapid, being essentially complete after 2 min. This was confirmed by the observation of equal amounts of U43 Cy3-riboSAM (fluorescent imaging) and the complete conversion of all U43 azido-riboSAM to U43 Cy3-riboSAM (UV imaging) at different reaction times (2, 4, 6, 8, 10, 20, and 30 min) (Figure 3D,E). These results demonstrate that the SPAAC reaction occurring on the riboSAM stem-loop was rapid and efficient, enabling the efficient incorporation of Cy3 into the U43 site.
Apart from the stem-loop, we sought to determine whether the SPAAC reaction on other structural regions of riboSAM would also be rapid and efficient. Next, we designed a new PLOR strategy to synthesize riboSAM with an azide group label at the U55 site of the internal loop (U55 azido-riboSAM) (Figure S4 and Table S3), and we similarly evaluated the yield of its reaction with DBCO-Cy3 to produce U55 Cy3-riboSAM (Figure 4A, left). Fluorescent gel imaging indicated that, similarly to that of U43, the production of U55 Cy3-riboSAM gradually increased as the DBCO-Cy3 concentration rose, reaching its maximum reaction yield at 500 times and above (Figure 4A,G). However, unlike the rapid reaction observed at U43, the reaction between U55 azido-riboSAM and DBCO-Cy3 required more than 6 min to complete (Figure 4B,H and Figure S5A,B). The crystal structure revealed that in the ligand-bound state, U35 of the truncated 55 nt riboSAM from the same family (corresponding to U55 in this study) is wrapped within the RNA interior (Figure S6) [23]. This suggests that the contact between U55 and DBCO-Cy3 may not be as direct and sufficient as that of U43, thus resulting in a slightly slower SPAAC reaction rate. We also examined the effect of the reaction temperature on the yield of SPAAC. Gel imaging indicated that the reaction efficiencies at 30 °C and 37 °C were significantly higher than that at 25 °C, especially at 37 °C (Figure S5C,D). Therefore, all SPAAC reactions in this study were conducted at 37 °C.
Furthermore, we conducted azide labeling at the U74 site (U74 azido-riboSAM) within the double-stranded region and the U84 site (U84 azido-riboSAM) within the single-stranded region of riboSAM (Figures S7 and S8 and Tables S4 and S5), and we detected the SPAAC reaction yields for U74/U84 azido-riboSAM (Figure 4C,E, left). Fluorescent gel imaging indicated that the production of U74/U84 Cy3-riboSAM also gradually increased as the DBCO-Cy3 concentration rose, reaching its maximum reaction efficiency when the DBCO-Cy3 concentration was 500–750 times higher than that of U74/U84 azido-riboSAM (Figure 4C,E,G). The reaction between U84 azido-riboSAM and DBCO-Cy3 was also completed within 2 min (Figure 4F,H). However, the difference lies in that the reaction rate between U74 azido-riboSAM and DBCO-Cy3 was significantly slower than that at other sites, with only half of the substrate reacting after 10 min and complete reaction not occurring until 90 min (Figure 4D,I). This may be due to the fact that U74 is located on a double helix and is buried inside the RNA molecule when riboSAM folds into a complex spatial structure, reducing its direct contact with DBCO-Cy3 compared to other sites (U43 on the stem-loop and U84 in a single-stranded region) (Figure S6). In summary, through ePLOR, we achieved efficient and rapid Cy3 labeling of specific sites in different structural regions of riboSAM.
2.2. Incorporation of Cy5 Fluorophore into Specific Sites of riboA Using ePLOR
On the basis of the results above, in order to confirm the effectiveness of ePLOR in fluorescently labeling a wider array of target RNAs, we utilized ePLOR for fluorescent riboA synthesis. Similarly, we first designed distinct PLOR strategies to introduce azide groups into the U22, U39, and U65 sites within the stem-loop, internal loop, and double-stranded regions of riboA, respectively, obtaining U22 azido-riboA, U39 azido-riboA, and U65 azido-riboA (Figure S9 and Tables S6–S8). Additionally, we verified the SPAAC reaction between azido-riboA and DBCO-Cy5 to ascertain whether Cy5, like Cy3, could be efficiently introduced into designated sites of riboA. Urea-PAGE detection revealed that, as the DBCO-Cy5 concentration increased, the production of U22/U39/U65 Cy5-riboA also gradually increased, similarly tending to reach the maximum reaction yield when the DBCO-Cy5 concentration reached 500–750 times (Figure 5A,C,E,G). This indicates that, compared to the reactions observed on riboSAM, the SPAAC reactions conducted on riboA exhibit equally high efficiency, facilitating effective fluorescent labeling of different structural regions of riboA through the application of ePLOR. Additionally, we used RP-HPLC to characterize the U22 Cy5-riboA generated under a condition with a 1000-fold molar excess of DBCO-Cy5. Similar HPLC spectra were observed to those obtained for U43 Cy3-riboSAM, demonstrating that the SPAAC reaction in riboA also achieved an almost perfect reaction of approximately 100% (Figure S10).
We also detected the reaction rates that occurred on different positions of riboA. Notably, the SPAAC reactions observed at the stem-loop (U22) and internal loop (U39) of riboA were significantly slower than those at the corresponding sites on riboSAM. Specifically, the reactions at U22 and U39 were completed after approximately 60 min (compared to just 2 min for U43 on riboSAM) and 50 min (compared to 6 min for U55 on riboSAM), respectively (Figure 5B,D,H). The crystal structure revealed that U22 is located within the kissing loop formed by loop2 and loop3, extending toward the interior of the RNA molecule, while U39 is positioned within the ligand-binding pocket, also extending inward and forming a hydrogen bond with the ligand adenine (Figure S11) [9]. This leads to some spatial hindrance for direct contact between the azide groups at these two sites and DBCO-Cy5. Similarly, U65 on the double helix extends toward the interior of riboA, analogous to the U74 site on riboSAM’s double helix, and requires 60–90 min to react completely (Figure 5F,H).
Source link
Yanyan Xue www.mdpi.com