3.1. Extraction Optimization
Table 1 shows the extraction yields of various extracts from different parts of P. nigrum (PNL) and P. betle (PBL) using different solvents. Based on the results, the influence of the solvent on the extraction yield followed the order methanol > aquadest > hexane, with the plant parts yielding in the order fruit > leaves > stems. For the PNL stem, methanol produced the highest yield of 5.35%, while hexane produced the lowest at 0.65%. For the leaves, methanol and hexane yields were 2.15% and 3.10%, respectively, with aquadest having a slightly higher yield at 3.15%. In terms of fruit, methanol provided the highest yield at 5.70%, and aquadest produced the lowest at 1.65%. For PBL, methanol had the highest stem yield of 4.10%, with hexane producing the lowest at 0.55%. The methanol extract was found to contain the highest amounts of hydroxychavicol, eugenol, and gallic acid. All three compounds were present at low levels in the P. betle leaf hexane extract [25]. The aquadest extract had the highest yield of 4.45%, while hexane had the lowest at 0.45%. Regarding fruit, methanol achieved the highest yield at 11.75%, followed by aquadest at 6.80%, while hexane provided the lowest at 2.70%. In general, methanol resulted in higher extraction yields across different plant parts for both species, especially for fruits. In a study by Rajopadhye et al. [26], black pepper roots were used for Soxhlet extraction with methanol producing a Peperine concentration of 9.56 ± 0.83 mg/g [26]. Hexane generally produced lower yields, while aquadest treatment was moderately effective, particularly for leaves and fruits. This information is crucial for selecting the most efficient solvent based on the desired yield and plant characteristics.
3.2. FTIR Profiling Analysis
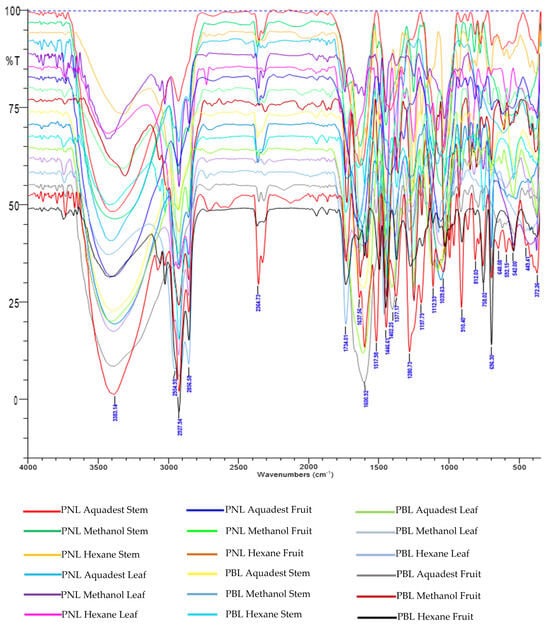
Figure 1 shows the FTIR spectral profiles of various active compounds isolated from Piper spp., including P. nigrum (PNL) and P. betle (PBL). The peaks at 3383.14 cm−1 and 3404 cm−1 represent OH stretching bonds, commonly associated with compounds such as piperanine from Piper retrofractum [27]. Furthermore, the peak at 3291.31 cm−1 corresponded to phenolic O–H stretching in hydroxychavicol from PBL [28]. The peak at 3469 cm−1 was related to the stretching bonds of OH groups in piperine from PNL [29]. The range of 2927.94 cm−1 to 2936 cm−1 suggested aliphatic C–H stretching bands associated with compounds such as hydroxychavicol [28] and β-caryophyllene oxide [30]. Moreover, the peak at 2856.58 cm−1 signified aliphatic C–H stretching in piperine and pellitorine [31]. Peaks at 1637.56 cm−1 and 1647.45 cm−1 indicated C=C symmetric aromatic stretching in hydroxychavicol [28] and piperanine [31], while peaks at 1658 cm−1 and 1636 cm−1 represent conjugated diene symmetric stretching in piperanine [27] and piperine [29]. Furthermore, peaks at 1600.92 cm−1 and 1613 cm−1 imply conjugated asymmetric diene stretching associated with piperine [32] and chavibetol [33,34]. The profile also included aromatic C=C stretching at 1582 cm−1 and 1598 cm−1, CH2 bending at 1446.61 cm−1, and C–O stretching at 1197.79 cm−1, representing various active compounds such as chavibetol [33,34], piperine [29], β-caryophyllene oxide [30], and hydroxychavicol [28].
This FTIR profile provides valuable insights into the functional groups and main components in Piper extracts, which are crucial for chemical characterization and phytochemical studies. CA based on functional groups detailed the hierarchical clustering process, in which observations were sequentially merged into larger clusters according to similarity and distance levels [18]. The analysis initially started with 18 distinct clusters, each representing individual observations or small groups. In the initial step, clusters 9 and 17 merged to form a new cluster (cluster 9) containing two observations, at a high similarity level of 99.8523%. This merging process continued, with clusters 14 and 15 combining into cluster 14 in subsequent steps, progressively reducing the total number of clusters. By the fifth step, clusters 3 and 6 merged into a larger cluster (cluster 3), now comprising four observations. This pattern further led to the aggregation of more clusters into increasingly larger groups, and each step reduced the number. For example, clusters 2 and 7 combined in the 14th step to form cluster 2, which included six observation groups.
In the final partition of the CA in Figure 2, six distinct clusters formed with high similarity levels >80%. Cluster 1 includes observations from the initial analysis step. Cluster 2 combines observations from the PNL methanol stem, PNL aquadest leaf, PBL aquadest stem, and PBL aquadest leaf. Cluster 3 aggregates observations from the PNL hexane stem, PNL hexane leaf, PNL fruit hexane, PBL methanol leaf, PBL hexane leaf, PBL aquadest fruit, and PBL methanol fruit, with Piperine as a significant component, and cluster 4 merged observations from PNL methanol leaf, PBL methanol stem, and PBL hexane fruit. Cluster 5 consolidates observations from clusters 7 and 12, while cluster 6 represents observations from cluster 8. The final grouping reflects the hierarchical relationships among observations, capturing similarities and distances throughout the clustering process [35]. Since clusters with similar functional groups based on FTIR data are likely to have the same pharmacological effects [36], representatives from each cluster were selected for further anti-MRSA activity testing.
This novel approach in natural product study simplifies the screening process by focusing on extracts with similar functional group compounds, thereby facilitating the identification of potential candidates without the need to test each extract individually. The extracts selected for anti-MRSA testing were PNL aquadest stem extract from cluster 1, PNL aquadest leaf extract from cluster 2, PNL fruit hexane extract from cluster 3, PBL methanol stem extract from cluster 4, PBL aquadest fruit extract from cluster 5, and PNL methanol fruit extract from cluster 6.
3.3. Anti-MRSA Activity
Based on Table 2, a one-way analysis of variance (ANOVA) was conducted to assess the anti-MRSA activity of seven samples. The ANOVA yielded a highly significant F-value of 122.76 and a p-value of 0.000, indicating notable differences in anti-MRSA activity among the samples at a significance level of 0.05. The samples included six extracts, which are PBL aquadest fruit extract, PNL aquadest leaf extract, PNL aquadest stem extract, PNL hexane fruit extract, PBL methanol stem extract, and ciprofloxacin as a control. The ANOVA provided an adjusted sum of squares of 736.57 and an adjusted mean square of 122.762, resulting in a high R2 value of 98.13%. This suggests that nearly all variability in anti-MRSA activity can be attributed to differences among the extracts. Additionally, the antimicrobial effectiveness of PBL was tested against 20 clinical isolates of S. pseudintermedius (10 MSSP and 10 MRSP) using the Kirby-Bauer disk diffusion method with P. betle extract disks at concentrations of 250, 2500, and 5000 µg. The minimum inhibitory concentration (MIC) for all isolates was determined to be 250 µg/mL [37].
Tukey’s pairwise comparisons provided further clarity on the results. Ciprofloxacin exhibited the highest mean anti-MRSA activity at 26 mm, significantly differing from all other extracts at a 99.58% confidence level. The PBL aquadest fruit extract had a mean activity of 19 mm, significantly outperforming the PNL hexane fruit extract (15 mm) and the PNL aquadest leaf extract (6 mm). The PBL methanol stem extract had a mean of 15 mm, showing significant differences compared to some lower-performing extracts, while the PNL aquadest stem extract (9 mm) also exhibited significant differences from the lowest-performing extracts. This study accumulatively reports the novel potential utility of the Curcuma longa L. and PNL extracts synergistically against MRSA infection by interfering with the mechanism of infectious angiogenesis and bactericidal action [38].
3.4. GC-MS Metabolite Characterization
The GC-MS analysis Table 3 of the Piper plant extracts showed important connections between the identified chemical components and anti-MRSA activity. The aquadest extract of PBL aquadest fruit extract and the PNL hexane fruit extract were subjected to GC profiling to identify the major compounds responsible for anti-MRSA activity. The hexane extract of PNL hexane fruit extract was found to contain piperine, a major component [39] with an area of 14.22% and a similarity index (SI) of 93%. This compound has strong antimicrobial properties and is capable of reducing the secretion of diverse virulence factors from MRSA. Therefore, piperine could be a potential antibiofilm molecule against MRSA-associated biofilm infections [40]. This correlates with the anti-MRSA testing results, where extracts containing piperine showed significant activity against MRSA. Pellitorine, also present in the extract with an area of 5.08% and an SI of 93%, may be able to inhibit bacteria efflux pumps. At 16 µg/mL, pellitorine increased the sensitivity of S. aureus (RN4220) to erythromycin through inhibition of the efflux pumps [41], thereby contributing to antimicrobial activity. Other components such as diisooctyl phthalate and piperanine, though present in significant amounts of 14.67% and 4.03%, respectively, require further investigation to understand specific roles in anti-MRSA activity [42]. For the PBL aquadest fruit extract, hydroxychavicol was identified as the primary compound, making up 81.89% of the extract with an SI of 93%. This suggests that the suggesting that the compound plays a crucial role in the effectiveness of the extract against MRSA.
The PBL leaf extract produced the highest percentage of hydroxychavicol content. Almost up to 90% of the final extract was found to contain hydroxychavicol based on HPLC analysis [43]. This supported the result that extracts from cluster 5, including hydroxychavicol, showed significant anti-MRSA activity. The PBL hydroxychavicol (36.02%) was the major constituent that inhibited the growth and biofilm formation of S. pseudointermedius and MRSP isolated from canine pyoderma in a concentration-dependent manner. Therefore, PBL is a potential candidate for the treatment of MRSP infection and biofilm formation in veterinary medicine [44]. Chavibetol, with an area of 12.01% and SI of 97%, also contributed to the anti-MRSA activity but was less dominant than hydroxychavicol. The PBL leaf extract (200 g) sliced into small pieces and subjected to hydro-distillation for 270 min was found to contain chavibetol (63.78%) [43]. The chemical composition of the ethanolic extract also contained chavibetol (12.03%) [44]. The anti-MRSA activity was correlated with the GC-MS results, demonstrating that extracts containing high concentrations of piperine and hydroxychavicol had stronger antimicrobial effects. This relationship underscores the significance of both compounds in anti-MRSA activity and their potential use in developing effective antimicrobial treatments.
3.5. Molecular Docking Analysis
Table 4 shows the binding free energy values and amino acid residues interacting with the MRSA target proteins 4CJN, 4DKI, and 6H5O [45] for the various tested compounds. These data provide insights into the molecular interactions between these compounds and MRSA target proteins, as well as how the results correlate with anti-MRSA activity assays and chemical profiles of Piper extracts identified through GC-MS.
The native ligand for the 4DKI target protein (Figure 3) has a binding free energy of −9.8 kcal/mol and interacts with the amino acid residues LYS 406, SER 462, ASN 464, GLN 521, and THR 600 through hydrogen bonds [46]. Ciprofloxacin, the positive control, has a binding free energy of −8.3 kcal/mol and interacts with the residues LYS 406, SER 462, and SER 643. The PNL hexane fruit extract, which contains piperine, has a binding free energy of −7.7 kcal/mol and interacts with the residues GLN 521, THR 444, GLY 402, and SER 400, indicating significant potential anti-MRSA activity. Piperine, as the major component with an area of 14.22% in this extract, significantly contributes to the antimicrobial activity. Additionally, Piperolein B (1.5%) showed a binding free energy of −8.3 kcal/mol, equivalent to that of ciprofloxacin, with interactions at residues LYS 406, SER 462, and ASN 464, further enhancing the potential anti-MRSA activity. Piperanine (4.03%) and diisooctyl phthalate (14.67%) also demonstrated significant binding free energies of 7.6 and 7.1 kcal/mol, respectively, with interactions at residues LYS406 and SER462. However, β-caryophyllene oxide and α-caryophylladienol did not show any H-bond interactions with this target protein, suggesting limited anti-MRSA activity.
For the 6H5O target protein (Figure 4), the native ligand has a binding free energy of −9.0 kcal/mol and interacts with the amino acid residues THR 444, SER 598, SER 461, SER 403, LYS 406, and ASN 464 [47]. Ciprofloxacin, as the positive control, has a binding free energy of −8.1 kcal/mol and interacts with LYS 406, SER 403, SER 462, and SER 643. Furthermore, piperine and piperanine from the hexane extract of PNL have a binding free energy of −7.3 kcal/mol and interact with residues SER 403, GLY 599, SER 598, THR 582, GLU 460, and ARG 445, indicating significant anti-MRSA activity supported by the major component diisooctyl phthalate. Minor components such as β-caryophyllene oxide, α-caryophylladienol, and piperolein B also contribute to the anti-MRSA activity of this plant extract, with binding free energies ranging from 6.8 to 7.1 kcal/mol. In the PBL extract, the major compounds hydroxychavicol (81.89%) and chavibetol (12.01%) show binding free energies of 5.4 and 5.6 kcal/mol, respectively, interacting with the residues LYS 406, SER 403, SER 462, GLY 599, ASN 464, and THR 600. This interaction indicates significant anti-MRSA potential, although lower than that of Piperine.
For the 4CJN target protein (Figure 5), the native ligand has a binding free energy of −7.2 kcal/mol and interacts with the residues LYS 273, ALA 276, and ASP 295 [48]. Meanwhile, ciprofloxacin has a binding free energy of −6.2 kcal/mol and interacts with LYS 273, LYS 316, and GLU 294. Piperine in the PNL extract has a binding free energy of −6.5 kcal/mol and a stronger interaction at residue 276 than the positive control. Piperanine shows a favorable binding free energy of −6.0 kcal/mol, with interactions at ASP 295, VAL 277, and GLN 292, supporting the anti-MRSA activity, along with β-caryophyllene oxide and diisooctyl phthalate, although piperolein B does not show an H-bond interaction with this target protein. Meanwhile, in the PBL extract, hydroxyychavicol and chavibetol showed binding free energies of 4.9 and 4.6 kcal/mol, respectively. The extract interacted with residues ASP 275, ASP 295, and GLY 296, indicating a contribution to anti-MRSA activity, although lower compared to PNL. The in vitro assays demonstrated that extracts containing major compounds such as piperine [40], diisooctyl phthalate, and hydroxychavicol [44] from clusters 6 and 5 were significantly effective in inhibiting MRSA growth. These results were consistent with the GC-MS results, which showed high concentrations of piperine (14.22%) and diisooctyl phthalate (14.67%) in the hexane extract of PNL fruit, as well as hydroxychavicol (81.89%) and chavibetol (12.01%) in the aquadest extract of PBL. Both proteins contribute to significant anti-MRSA activity. Docking analysis showed that these compounds strongly bind to key residues in MRSA target proteins, reinforcing their potential as effective antimicrobial agents.
Source link
Budiman Yasir www.mdpi.com