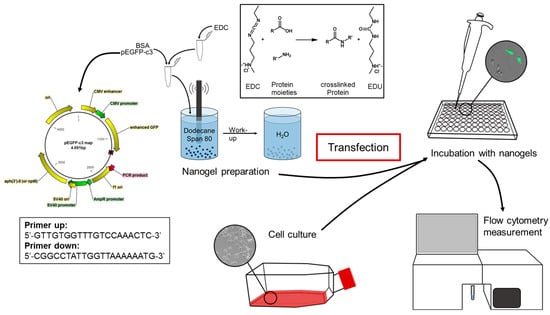
The uptake of nucleic acids into living cells with the aim to provide them with extra genetic information, e.g., for the recombinant production of foreign proteins, is an essential experimental task in cell biology. A variety of tools and techniques for this process known as transfection has been invented in recent decades [7,42]. In addition to chemical compounds as transfection facilitators like cationic polymers [14], calcium phosphate [16], or cationic lipids [15], a category in which the reference transfection agent Lipofectamine used here can be subsumed, different classes of nanoparticles have been described including gold nanoparticles [43], organically modified silica (ORMOSIL) nanoparticles [42,44], biologically degradable polymer nanoparticles (i.e., poly(lactic acid)) [45]; poly(β-amino ester) [46]), or chitosan-based nanoparticles [47]. With the recent introduction of inverse-nanoemulsion-derived BSA nanohydrogels, we have added a simple protein-based nanoparticle to the available portfolio of cell uptake-mediating nanostructures [34]. The existing robust and simple synthesis protocol for these nanogels was shown here to allow for easy experimental modification by simply adding increasing amounts of plasmid DNA as an example of functional nucleic acids without disturbing the nanogel quality delivering indistinguishable key parameters like size, swelling, agglomeration behavior, and zeta-potential. These NanoTrans-gels can be stored in a ready-to-use manner in simple TRIS·HCl buffer (pH 7.4) at 4 °C, and addition to the target cells allows for conditions resembling those of standard transfection protocols as they are used, e.g., with Lipofectamine (i.e., incubation time, temperature, expression time, etc.). The principal functionality of transfection with calcium phosphate nanoparticles has been shown, but a large study with a variety of cells found that this method resulted in notoriously low reproducibility of transfection efficiencies generally below those obtained with the commercial DOSPA/DOPE transfection agent Lipofectamine 2000 [24,48]. Lipofectamine has been optimized with the introduction of Lipofectamine 3000, which is a kit that contains a lipid mixture and a so-called P3000 reagent with a composition that is not fully known. However, this has been claimed by the manufacturer and described in a series of studies to improve transfection efficiencies for a range of different cell lines including “hard to transfect” cells like A549 [49,50,51,52,53]. In the context of such a technological breakthrough, it is of special remarkability that the inverse-nanoemulsion-derived BSA/EDC nanohydrogels, as carriers of DNA in our preliminary study using, so far, protocols that are not further optimized, instantaneously allowed for improvements in A549 transfection efficiencies of up to 95-fold compared to Lipofectamine 3000/P 3000 dependent protocols as the reference. Differences in transfection efficiencies principally depend on genetic and cell physiological conditions and are influenced by a vast variety of parameters that are hard to control in experiments. The respective nucleic acids have to cross the cell membrane, escape from the lysosome, and then enter the nucleus thereby—most importantly—avoiding their degradation [54,55,56,57,58,59,60,61]. Without yet having strong experimental evidence for increased protection of the nucleic acid when it is on the way from the membrane to the expressed marker gene(s), the speculation appears to be reasonable that DNA molecules tightly cocooned in a protein-based hydrogel may be stored extremely safely against (enzymatic) degradation like in a molecular secure container. If degradation-prone inactivation of functional vectors in a hard-to-transfect cell line like A549 is a crucial parameter, in turn, a hydrogel-mediated stabilization may explain the drastic effect observed for this cell line. However, the particle uptake, the release of cargo DNA, and its fate until the expression of the transfected phenotypes require more detailed analyses, which will be part of a follow-up study also involving more cell lines and different cargo DNAs. Deeply appreciating the common perception that the complex physiological process of transfection requires optimized conditions for every potential target cell line [62,63], we dare to hope that the NanoTrans-gel concept may develop into a broadly applicable, novel, and valuable expansion of the portfolio of transfection technologies available in cell biology.
Source link
Michael Kohler www.mdpi.com