1. Introduction
Subjective tinnitus is the perception of a phantom sound, negatively affecting more than 20% of the world’s population. Multiple risk factors, including head and neck injuries, otologic diseases, and certain medications, can induce tinnitus; however, occupational- or leisure-related sound overexposure is the most common trigger [
1]. A variety of treatments are available to help people adapt to and modulate their tinnitus perception, but no proven treatments that reliably eliminate tinnitus itself have been established at present. Additionally, there is no single drug that has been approved by the Food and Drug Administration (FDA) or European Medicines Agency (EMA) on the market targeting tinnitus. Understanding the mechanisms behind tinnitus perception may help to develop strategies for effective treatment.
The mechanisms for the genesis of tinnitus are complex and still not entirely understood, but it is believed to involve neuronal hyperactivity developed at several neural stations along the auditory pathways. Numerous studies have investigated the involvement of the cochlear nucleus (CN), the first auditory nucleus in the central auditory system, in the development of tinnitus, especially in the dorsal cochlear nucleus (DCN) [
2]. The earliest study reported that the loudness of tinnitus could be changed by direct electrical stimulation of the DCN [
3]. Subsequently, neural hyperactivity has been observed in the DCN in tinnitus animals [
4,
5,
6], and the DCN has also been found to exhibit several forms of neural plasticity that parallel the traits of tinnitus [
7], including injury-induced plasticity, temporal plasticity, stimulus-dependent plasticity, and modulatory plasticity. However, the fact that DCN lesions prior to acoustic trauma could prevent tinnitus generation, but failed to reverse established tinnitus in rats [
8,
9], suggests that although the DCN may be essential in tinnitus generation, it may not be necessary for chronic tinnitus maintenance and perception.
On the other hand, the auditory cortex (AC), which is the last auditory region in the ascending auditory pathways, has been suggested to play a role in the generation and perception of tinnitus. For example, an increased spontaneous firing rate and synchrony as well as tonotopic reorganization have been reported in the AC of noise-induced tinnitus animals [
10,
11,
12,
13], and stimulation of the AC suppressed tinnitus in both animals [
14] and humans [
15]. Moreover, animals with behaviourally confirmed tinnitus were found to exhibit a decreased inhibitory synaptic transmission in the tinnitus frequency region of the AC, and the reduced inhibitory synaptic transmission was correlated with a reduced expression of the GABA-synthesizing enzyme, glutamic acid decarboxylase, in the same region [
16]. Furthermore, the systemic administration of a GABA-enhancer drug (an inhibitor of GABA transaminase), vigabatrin, 15 min before the tinnitus behavioural testing, was able to abolish tinnitus perception in such animals [
16].
Therefore, neurochemical alterations within the auditory brain regions might be one of the factors underlying tinnitus generation. To date, most of the neurochemical studies in tinnitus research focus on the changes in amino acids, as they are the most abundant neurotransmitters in the central auditory system [
17]. For example, glutamate increased in the ventral cochlear nucleus (VCN) at 1 week but decreased at 2 weeks then recovered at 3 months after noise exposure in chinchillas [
18], and elevated aspartate, glutamate, and GABA and reduced taurine concentrations were found in the CN and the AC of hamsters at 5 months, following intense sound exposure [
19]. In addition, taurine, serine, threonine, and alanine were also found to decrease with a long-term (1–1.5 months), but not a short-term (2 h or 1 day), time point after noise exposure in the CN [
20]. However, none of these studies measured amino acids in the extracellular space, even though variations in extracellular levels may correlate with changes in neuronal activity such as neurotransmissions. In a recent study from our laboratory, time-dependent effects of acoustic trauma and tinnitus on extracellular levels of amino acids were investigated in the inferior colliculus (IC) of rats using in vivo microdialysis [
21]. Although acoustic trauma caused a significant increase in GABA up to 1 week post-acoustic trauma, there was no significant change in GABA levels at 5 months post-acoustic trauma in tinnitus animals [
21]. To the best of our knowledge, the present study is the first time extracellular amino acid levels have been measured in the CN and the AC simultaneously in the same rats using in vivo microdialysis and compared between sham and tinnitus-positive groups.
2. Materials and Methods
All procedures were approved by the University of Otago Committee on Ethics in the Care and Use of Laboratory Animals (approval number: AEC#19/2011; approval date: 1 February 2020). All methods were performed in accordance with the relevant guidelines and regulations. We confirm that this study is reported in accordance with the ARRIVE guidelines (
https://arriveguidelines.org, accessed on 1 March 2020).
Thirty-two male Wistar rats, aged between 8–10 weeks (300–350 g at the beginning of the experiment), were obtained from the Hercus Taieri Resource Unit, University of Otago, Dunedin, New Zealand. The rats were housed in pairs and maintained in an animal facility with a 12/12 h light/dark schedule under a regulated temperature (21 ± 1 °C) and humidity (55 ± 5%). All animals were given ad libitum access to food and water, except when they had restricted access to water throughout the behavioural test for tinnitus.
Due to the quarantine period of COVID-19, the study was conducted on two batches of rats with a 2-month age difference between them, making age a necessary factor to be considered in this study. Therefore, thirty-two rats were randomly allocated into four groups: (1) sham aged,
n = 6; (2) sham young,
n = 6; (3) exposed aged,
n = 10; and (4) exposed young,
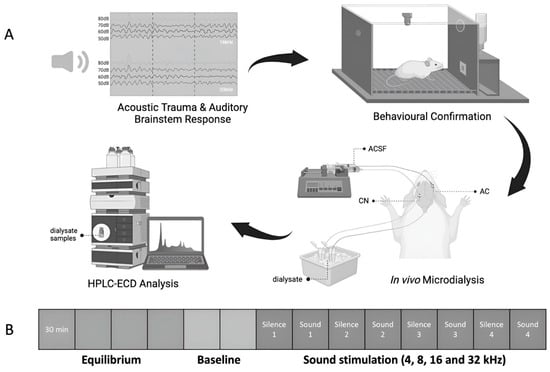
n = 10. The animals received either acoustic trauma or the sham procedure under anaesthesia, and hearing levels were measured before and immediately after the acoustic trauma or sham procedure using acoustic brainstem-evoked responses (ABRs). One month later, they were assessed for the perception of tinnitus using a conditioned lick-suppression paradigm, which took 4–6 weeks to complete. One week after the completion of the tinnitus testing, the ABR was measured again to verify the recovery of hearing levels. Subsequently, in vivo microdialysis experiments were conducted, and the dialysate samples were collected simultaneously from the CN and the AC of the same animal under anaesthesia. The levels of amino acids in the microdialysate were quantified using high performance chromatography (HPLC) coupled with an electrochemical detector (ECD) (
Figure 1A).
Unilateral acoustic trauma was delivered using the techniques previously described by Bauer and Brozoski [
4] and our previous publications, e.g., [
21,
22,
23]. The rats were anaesthetised with a ketamine (75 mg/kg and 100 mg/mL, s.c.) and dormitor (0.3 mg/kg and 1 mg/mL, s.c.) mixture, prior to the sham procedure or acoustic trauma. A pure 16 kHz tone with an intensity of 115 dB (RZ6 multi-I/O processor, Tucker-Davis Technologies, Alachua, FL, USA) was delivered to one of the ears for 1 h through a closed-field magnetic speaker with a tapered tip (Tucker-Davis Technologies) attached to a 3 mm cone-shaped speculum that fitted tightly into the external auditory canal. Acoustic values were calibrated before acoustic trauma by a pre-polarised free-field microphone (Type 40 BE, GRAS Sound & Vibrations, DK-2840 Holte, Denmark). The unexposed ear was plugged with a foam earplug during the sound exposure. To reduce lateralisation bias, the noise exposure was counterbalanced between the left and right ears. The sham animals received an identical anaesthesia procedure and duration as the acoustic trauma animals, but without noise exposure, in the same sound-attenuated chamber.
Acoustic brainstem-evoked responses (ABRs) were measured in all rats in both ears before and immediately after acoustic trauma. Using an identical anaesthesia technique and setup as described above, three stainless-steel subdermal needle electrodes (14 0.38 mm, Technomed) were inserted subcutaneously at the vertex and over the bullae below the recording ear, and the same position below the other ear was used for the reference electrode, and at the occiput, as the ground electrode. Tone bursts of a 5 ms duration (2 ms rise/decay and 1 ms plateau), presented at a rate of 21/s, were employed to examine the ABR thresholds of the animals. These tone bursts were presented at four different frequencies (8, 16, 20, and 32 kHz, respectively) in a series of decreasing intensities, starting with a level that elicited an observable evoked potential. Hearing thresholds were defined as the lowest intensity that produced a visually distinct potential, progressing in 20, 10, and 5 dB steps. At the end of the ABR measurement, the anaesthesia was reversed by an injection of antisedan (1 mg/kg and 5 mg/mL, s.c.).
The presence of tinnitus was assessed by a conditioned lick-suppression paradigm, as described in our previous publications, e.g., [
21,
22,
23]. Briefly, the animals were subjected to water restriction and were allowed to drink in an operant conditioning testing chamber during behavioural testing. The conditioned lick-suppression paradigm consisted of 15 min of testing, and the animals went through 3 phases, including acclimation, conditioned lick-suppression training, and frequency discrimination. A constant 60 dB SPL broad band noise (BBN) was played as a background noise throughout each 15 min session except at 10 intervals, at which point 15 s acoustic stimuli presentations or silence was randomly allocated (
Figure 1B). Two of the 10 presentations were always speaker-off periods (i.e., silence), and the remaining 8 were either BBN, 20 kHz tones, or 32 kHz tones at 4 different intensity levels (40, 50, 60, and 70 dB SPL for BBN; 60, 70, 80, and 90 dB SPL for 20 kHz; and 70, 80, 90, and 100 dB SPL for 32 kHz) in a random order, with each stimulus presented twice within each session. The stimulus was not delivered in the first or last minute of the session. The type of stimulus varied between sessions but remained constant within a session. The animals had 3 sessions of acclimation for each type of stimulus before moving to the conditioned lick-suppression training, where the acoustic stimulus was presented in the same way as acclimation, except that a 3 s mild foot shock (0.3–0.45 mA) was presented at the end of each speaker-off (silence) period. The foot shock acted as an unconditioned stimulus, and the animals learned the association between the speaker-off period and the foot shock by suppressing the licking during the silence period. The number of licks during the 15 s preceding the stimulus presentation and during the 15 s stimulus-presentation period were recorded using an electronic photo beam. The lick-suppression ratio was calculated by comparing the number of licks between these two periods:
Once a stable lick suppression was established (SR < 0.2), the animals underwent a frequency discrimination phase. The acoustic stimuli presented in the discrimination phase were identical to the previous phases, except that the animal received a mild foot shock only when their SR ratio was 0.1 or above during the speaker-off period. Frequency discrimination curves were composed based on the SRs for each acoustic stimulus presented.
The animals were anaesthetised with urethane (1.5 g/kg, i.p.), and the body temperature of the animals was maintained at 37.0 ± 0.5 °C using a thermostatically regulated heating pad (Harvard Apparatus, Holliston, MA, USA) throughout the procedure. The animal was secured to the stereotaxic frame using hollow ear bars, and xylocaine (0.1 mL, with 1:100,000 adrenaline) was injected, s.c., along the incision line. An incision was made along the midline of the head, and the periosteum was removed to expose the skull. Two small holes were drilled to allow the implantation of a microdialysis guide cannula into the primary AC (ML = 6.8 mm, AP = −4.5 mm, and DV = 4.5 mm) and the CN (ML = 3.8 mm, AP = −11.3 mm, and DV = 7.8 mm), respectively, using the Paxinos Rat Brain Atlas [
24]. The microdialysis probes were then inserted into the guide cannula to collect the dialysate samples. Throughout the course of sample collection, 3 mL of saline (0.9% NaCl; Baxter International Inc., Auckland, New Zealand) was applied, s.c., every 4 h in order to prevent dehydration, and 0.5 mL of urethane was supplemented every 4 h to keep the animal under anaesthesia. The toe-pinch reflex, body temperature, and corneal dryness were monitored continually throughout the surgery.
Initially, a 2 h equilibration period established a stable baseline after probe insertion. Subsequently, two baseline dialysate samples were collected every 30 min. Afterwards, sound stimulation was delivered for 30 min at each frequency (4, 8, 16, and 32 kHz; at 75 dB SPL; 5 pulses/s, with a pulse duration = 5 ms and with a 1 ms rise/fall time) in the ear which had been exposed to acoustic trauma or the sham procedure beforehand (
Figure 1B). Each sound-stimulation period was separated by a 30 min silence period. Samples were stored in a −80 °C freezer until high-performance liquid chromatography (HPLC) analysis.
The mobile phase contained a 28%-methanol, 2%-acetonitrile, and 100 mM di-sodium hydrogen phosphate anhydrous (Na2HPO4, VWR Chemicals) buffer in MilliQ water, with the pH adjusted to 6.75 using HPLC-grade orthophosphoric acid (Fisher Scientific, Loughborough, UK). Ten μL of the internal standard (homoserine, 1 ng/µL) was added to the amino acid standards or the samples, followed by a pre-column derivatisation reaction, which was achieved by mixing 40 μL of the o-phthalaldehyde (OPA) (Sigma, Tokyo, Japan) with the standards or samples and incubating for 3 min. Fifty µL of the mixture was injected into the HPLC system, and the derivatised amino acids were detected by the ECD using the following settings: a guard-cell potential of +650 mV with a gain range of 100 nA, an E2 potential of +200 mV with a gain range of 100 μA, and an E3 potential of +700 mV with a gain range of 500 nA. The amino acids were identified based on their retention times and quantified according to the standard curve generated by the Chromeleon 7.3 software.
A statistical analysis was conducted on the ABR thresholds, frequency discrimination curves, and amino acid levels in response to sound stimulation using SPSS 27 (Chicago, IL, USA). The baseline levels of amino acids were analysed using GraphPad Prism v.9.5.1 (Boston, MA, USA). All data were tested for the assumptions of normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. The non-normal data were square root (sqrt) values or log-transformed to meet the criterion for a statistical analysis.
A linear mixed model (LMM) analysis, using a restricted maximum likelihood procedure, was carried out in preference to repeated measure ANOVAs because of the problem caused by extensive autocorrelations in repeated-measure data. LMM analyses model the covariance structure of the repeated-measure data in order to address this problem [
25]. Akaike’s Information Criterion (AIC) estimates the goodness of fit of a given model, and the most appropriate covariance matrix structure was used based on the smallest AIC value. Using LMM, the ABR data were analysed with exposure (sham or exposure) and age (aged or young) as the between-group fixed factors, with frequency (8, 16, 20, or 32 kHz); side (ipsilateral or contralateral); and time (pre-acoustic trauma, immediately post-acoustic trauma, or 3 months following acoustic trauma) as the repeated measures. The tinnitus testing data were analysed with group (sham or tinnitus-positive) and age (aged or young) as the between-group fixed factors and intensity (0, 40, 50, 60, and 70 dB SPL for BBN; 0, 60, 70, 80, and 90 dB SPL for 20 kHz; and 0, 70, 80, 90, and 100 dB SPL for 32 kHz) as the repeated measures. Before the analysis of the amino acid data, the Grubbs’ test was conducted to detect and remove the outliers in a univariate data set that followed an approximately normal distribution, with alpha < 0.01. The basal amino acid data were analysed using a two-way ANOVA, with group (sham and tinnitus-positive) and age (aged and young) as the fixed factors. The amino acid data, in response to external sound stimulation, were expressed as a percentage of the corresponding sound-off period preceding each sound-on period and were analysed using an LMM analysis, with group (sham and tinnitus-positive) and age (aged and young) as the between-group fixed factors and frequency (4, 8, 16, and 32 kHz) as the repeated measures. For all data, statistical significance was set as
p ≤ 0.05.
The animals were euthanized at the end of the in vivo microdialysis experiment, and brains were removed and placed in 10% phosphate-buffered formalin at 4 °C for 3 days, followed by 0.1 M phosphate buffer (PB) containing 30% sucrose at 4 °C until the brain sank. Brains were then embedded in an optimal cutting temperature (OCT) compound and sectioned and stained with cresyl violet. The placement of the microdialysis probes was examined using a Microfiche Reader. Partly due to the disruptions of COVID-19, which entailed isolation for 2 months in New Zealand, only a few samples of the brains were analysed histologically, although this was counterbalanced and randomized across groups (i.e., the brains which were not analysed were Missing at Random (MAR)), which means that the histological analysis was not biased toward any single group [
25].
4. Discussion
A pure tone of 16 kHz at 110 dB SPL was delivered to rats unilaterally for 1 h in the present study, which caused an acute hearing loss in the exposed ear of acoustic trauma-exposed rats across all the frequencies tested. This observation of hearing loss after acoustic trauma was generally consistent with our previous findings [
21,
22,
23,
26]. In order to avoid secondary sound trauma to the animals’ ears, the highest intensity of the tone bursts used for all frequencies was no more than 90 dB SPL during the post-exposure ABR threshold tests in the current study. Therefore, the maximum 90 dB SPL post-exposure ABR threshold measured might not represent their actual hearing thresholds following acoustic trauma. Nevertheless, the degree of hearing loss at 16 kHz, 20 kHz, and 32 kHz was significantly greater than that at 8 kHz. This is thought to be the consequence of non-linear basilar mechanics, resulting in a larger amplitude of basilar membrane motion in response to the acoustic trauma frequency [
27]. ABR thresholds were measured again after the behavioural confirmation of tinnitus, which was at 3 months following exposure to acoustic trauma. It was unexpected to see that the ABR thresholds completely recovered in the exposed ears of acoustic trauma animals in the aged group but not in the young group, as ABR thresholds have been shown to completely recover at 5–6 months following acoustic trauma in our previous studies, e.g., [
21,
22,
23]. This discrepancy cannot be simply attributed to the age difference, since the age of the animals in the young group, at the time of acoustic trauma, was very similar to those animals used in our previous studies, e.g., [
21,
22,
23]. A close inspection of the data revealed that, among the ten acoustic trauma rats in the young group, ABR thresholds remained elevated in three tinnitus-positive rats, while they returned to a normal level in four tinnitus-positive rats and three tinnitus-negative rats. This suggests that there might be some individual differences in the speed of recovery following acoustic trauma. It is also noteworthy that the last ABR thresholds were measured at 3 months after acoustic trauma in the present study, while they were measured at 5–6 months after acoustic trauma in previous studies, which may account for the incomplete recovery in the present study. Nevertheless, the elevated ABR thresholds in the exposed ear of the 3 rats should not have affected the tinnitus behavioural testing, as hearing in the unexposed ear was intact. The fact that both hearing-loss and hearing-intact animals exhibited behavioural evidence of tinnitus further supports the view that gross changes in ABR thresholds may not always accompany tinnitus, and a “hidden hearing loss” could be present with a normal audiogram [
28].
In the present study, the tinnitus induction rate was 70%, which is inconsistent with the tinnitus induction rate of 30% to 80% reported in previous studies, e.g., [
21,
22,
23]. In addition, the tinnitus perceived by these rats had acoustic features resembling 20 kHz and 32 kHz tones, which is in agreement with previous findings, e.g., [
21,
22,
23]. Furthermore, an unexpected significant age effect was observed during tinnitus assessment, i.e., the young rats showed greater lick suppression than the aged rats following acoustic trauma. Given that the tinnitus induction rate was the same in both groups, the difference in the degree of lick suppression cannot be explained by age-related differences in tinnitus development. One possibility for the greater lick suppression in the young tinnitus animals could be that they experienced louder tinnitus than the aged animals, or they had the co-existence of hyperacusis, which might have made the tinnitus-resembling tones easier to be heard. Although hearing loss in the young animals in the present study was more persistent than that in the aged animals, there is no evidence to support a direct link between hearing loss, tinnitus loudness, and hyperacusis, and further investigations are needed to better understand the underlying mechanisms [
29].
Increases in neuronal activity have been reported in the CN and the AC after acoustic trauma [
4,
30,
31]. It has been suggested that the hyperactivity might be due to an imbalance between excitatory and inhibitory neurotransmissions [
30,
31,
32]. Since extracellular levels of neurochemicals are believed to more closely reflect neurotransmissions and/or neurochemical function than the neurochemical levels in tissue homogenates [
33,
34], in vivo microdialysis is regarded as one of the best methods to monitor changes in neurotransmission. However, only two studies have investigated the relationship between the extracellular level of neurochemicals and tinnitus development. The first one, by Liu and colleagues [
35], assessed serotonin release in the inferior colliculus (IC) and the AC of rats after salicylate administration using microdialysis. They found that serotonin levels increased to 268% of the baseline in the IC and 277% of the baseline in the AC at 2 h and 3 h after salicylate injection, respectively. The second study investigated time-dependent changes in amino acids in the IC of rats following acoustic trauma and tinnitus confirmation using microdialysis [
21], which reported an increase in GABA levels a few hours and at 1 week after acoustic trauma. However, no changes in the basal levels of amino acids were found in tinnitus-positive animals 5 months later. The present study has the advantage of implanting two microdialysis probes in the CN and the AC, respectively, in the same rat, which made it possible to directly compare the extracellular levels of amino acids in the CN and the AC in relation to chronic tinnitus induced by acoustic trauma. We found no significant differences in any of the amino acids examined between sham and tinnitus-positive rats in either the CN or the AC during the baseline period, which is consistent with the findings of the IC using the same methods [
21]. The lack of difference between the sham and tinnitus-positive animals was also reflected in the animo acids’ response to sound stimulation, where none of the amino acids examined responded differently between the two groups in either the CN or the AC.
Interestingly, serine levels were found to be lower in the aged animals in the AC in both the sham and tinnitus-positive groups, while the lowered level of threonine in the aged animals was only observed in the sham group. A general age-related decrease in amino acid levels, including serine and threonine, in different brain regions has been reported by comparing two groups of rats with a 26-month difference in age [
36]. It is surprising to see that an age difference also existed in our current study, where there was only a 2-month difference between the young and aged groups. In the case of threonine, this age-related difference was only observed in the sham group. Since there was no difference in the ABR thresholds between the two age groups in the sham animals, the present results cannot be simply explained by age-related hearing loss [
37]. In addition to the age-related difference, threonine and glycine levels in the CN varied in response to different frequencies of sound stimulation, and, in particular, the response was higher at the 4 kHz tone than the 16 kHz tone, which might be due to the variation in the placement of the microdialysis probes in different tonotopic regions of the CN.
Nevertheless, using microdialysis, the present study found that the presence of tinnitus did not affect the extracellular levels of amino acids, nor the amino acids’ response to sound stimulation, in either the CN or the AC in animals. Although the results do not support the view that tinnitus is generated by an imbalance between excitatory and inhibitory neurotransmissions [
19,
38,
39,
40,
41], the findings are consistent with a microdialysis study of the IC [
21], which reported no difference in amino acid levels between sham, tinnitus-negative, and tinnitus-positive animals. Further studies investigating a wide range of neurochemicals in both auditory and non-auditory brain regions are needed in order to understand tinnitus-related changes in neurotransmission.