1. Introduction
In controlled environment agriculture (CEA), lighting is a pivotal factor that profoundly influences the overall performance of plants [
1]. Photosynthetically active radiation (PAR) refers to an essential electromagnetic spectrum for plant photosynthesis that ranges from 400 to 700 nanometers in wavelength [
2]. During the process of photosynthesis, plants transform light energy into chemical energy, which drives their growth, development, and productivity [
3]. PPFD (photosynthetic photon flux density) is expressed as micromoles of photons in the visible electromagnetic range (PAR 400–700 nm) that fall on a unit of area per time unit, expressed in µmol m
−2 s
−1. Daily light integral (DLI) represents the cumulative amount of light in the PAR region that plants receive within a 24 h period and is expressed in moles of photons and incident per square meter per day (mol m
−2 day
−1).
Light intensity, expressed as PPFD, is one of the top measurements in CEA, which directly influences plant photosynthetic productivity and OPEX [
4,
5]. From a physiological point of view, light can only be absorbed and used efficiently by plants until the saturation point [
6,
7]. Beyond this threshold, additional light may disbalance plants’ energy supply and consumption [
8]. Moreover, lighting levels above optimal plant requirements affect the profitability of any indoor farming facility.
It was proven that the biometric parameters of plants directly depend on light intensity [
9]. Numerous studies have been conducted to find optimal light intensity for maximum biomass gain, and they provide varying results. For instance, optimal PPFD levels for basil growth were reported to be 250 µmol m
−2 s
−1 (DLI 14.4 mol m
−2 d
−1 [
10], 224 µmol m
−2 s
−1 (DLI 12.9 mol m
−2 d
−1 [
11]), 500 µmol m
−2 s
−1 (DLI 28.8 mol m
−2 d
−1 [
12], or even 600 µmol m
−2 s
−1 (DLI 38.9 mol m
−2 d
−1) [
13]. In vertical farming, maximum biomass gain was achieved in lettuces cultivated under 300 µmol m
−2 s
−1 (DLI 17.3 mol m
−2 d
−1) [
14]. Biomass accumulation is the main priority for any indoor facility and is specific to crops and other cultivation conditions. Moreover, various produce quality parameters can also be influenced by PPFD manipulations, e.g., nitrate content tends to decrease with increasing light intensity [
15]. Furthermore, an appropriate light strategy can reduce nitrate content without diminishing the yield [
16] but increase soluble sugar and vitamin content [
17]. The plant antioxidant system response is dependent on light intensity [
18]. Excess photon energy from higher light intensity boosts the capacity of the antioxidant system. Consequently, exposure to high light intensity can enhance the quality of lettuce [
19]. Multiple research studies show that main artificial illumination properties, such as photoperiod, spectrum, and intensity, can alter vegetable quality in multiple ways: yield, mineral nutrition, photosynthetic, antioxidant response, soluble sugar, and nitrate content. Moreover, the cultivation conditions were proven to affect the post-harvest quality of cultivated greens and their preservation [
20,
21]. While most knowledge is published on lettuce and basil, this study is dedicated to
Perilla frutescens, a plant that has the potential to expand the portfolio of CEA-cultivated plants. Perilla and basil belong to the
Lamiaceae family and perform the C3 photosynthesis pathway [
22].
Perilla frutescens contains various compounds beneficial for humans, such as phenolic acids, flavonoids, essential oils, rich in perillaldehyde, triterpenes, polysaccharides, carotenoids, phytosterols, fatty acids, tocopherols, etc. [
22,
23]. Perilla is widely consumed in Asian countries as a culinary herb and traditional medicine [
24]. Previous studies primarily analyzed the effects of the light-emitting diode (LED) lighting spectrum on perilla growth and phytochemical content in CEA. They determined that monochromatic red light (661 nm, 230 µmol m
−2 s
−1) improved root and shoot fresh weight, leaf area, and photosynthetic rate, as well as total phenolic content, antioxidant capacity, rosmarinic acid content, and caffeic acid content, both in red and green perilla cultivars, compared to monochromatic blue (447 nm) and combinations of red and blue light [
25]. UV-A (365 nm) and deep-blue (415 nm) light, supplemented for red, blue, and white LEDs, did not promote the growth of green perilla. However, supplemental deep-blue light resulted in higher levels of total phenolic compounds, antioxidant capacity, rosmarinic and caffeic acids, and perillaldehyde in red and green cultivars. Both supplemental UV-A and deep-blue light increased the total anthocyanin content [
26]. Lighting PPFD effects for perilla plants in a controlled environment were less explored. Experiments using cool white, fluorescent lamp lighting show that leaf net photosynthetic rates were raised in red and green perilla cultivars when PPFD increased from 100 to 300 µmol·m
−2·s
−1 [
27,
28,
29]. The highest apigenin contents were determined in green perilla at 200, while in red at 300 µmol·m
−2·s
−1 [
28]. Notwithstanding, there is a gap in the knowledge of the effects of LED lighting intensity on perilla plants in CEA. Following that, this study aims to evaluate the impact of cultivation lighting PPFD on the productivity and quality of perilla plants at harvest and during their post-harvest storage.
2. Materials and Methods
Cultivation conditions. Cultivation experiments were conducted in a walk-in controlled environment chamber. In the chamber, a day/night temperature of 21/17 ± 2 °C with a 16 h thermoperiod and photoperiod was maintained. The relative humidity was kept at 50–60%, and the ambient CO2 concentration was maintained at 1000 ppm. Tunable LED lamps (TU-AS GTR 2V 0021096109 C1 DL ST, Tungsram, Budapest, Hungary) were used to provide artificial lighting with a spectral composition of 61% deep red, 20% blue, 15% white, and 4% far red. These proportions were consistent across all light-intensity treatments. The photosynthetic photon flux density (PPFD) was measured and controlled at the top of the plant (photometer-radiometer RF-100, Sonopan, Bialystok, Poland). The seeds of green-leaf perilla (Perilla frutescens L.; CN Seeds, Ely, UK) were sown in rockwool cubes (20 × 20 mm, Grodan, Roermond, The Netherlands); one seedling per rockwool cube and cultivated under PPFD of 200 µmol m−2s−1. Ten days after germination, the seedlings were transplanted into 40 L deep water culture (DWC) hydroponic tanks (12 pots per tank, creating 50 plant m−2 cultivation density), and lighting treatments started. For hydroponic nutrient solution, equal parts of Hydro a (NPK 3-0-1, Ca 4.2%, MgO 0.4%) and Hydro b (NPK 1-3-6, MgO 1.4%) commercial concentrate (Plagron, Ospel, The Netherlands) were mixed with deionized water (ratio 1:400 for each component). Nutrient solution pH at preselected interval of pH 5.5–6.0 was actively maintained using acid-base titration.
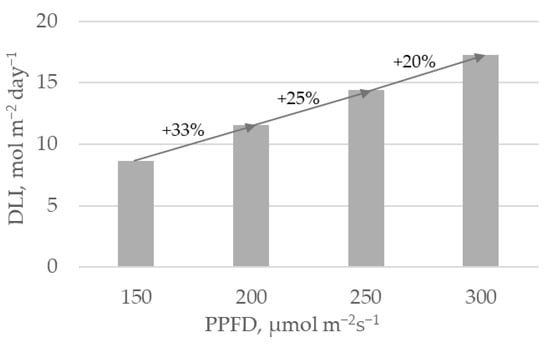
Lighting intensity treatments. Plants were illuminated with four lighting PPFD levels of 150, 200, 250, and 300 ± 10 µmol m
−2s
−1, constituting daily light integrals (DLIs) of 8.64, 11.52, 14.4 and 17.28 mol m
−2d
−1 per 16 h, respectively, for four weeks after transplanting (
Figure 1).
Plants containing 5 to 6 fully developed leaves were harvested, and all above-ground plant material was used for analysis. At harvest, biometric measurements and biochemical analyses were performed, while part of the harvested plants were stored in the dark at +6 °C temperature in plastic containers (15 × 20 × 5 cm). Biometric measurements and biochemical analyses were performed 1, 3, and 5 days of post-harvest storage.
Experimental design. Each lighting treatment was performed in 3 parallel experimental replicates. For biometric measurements at harvest and storage, three plants were randomly selected from each treatment replication (9 plants per treatment). The same plant was weighed before and after storage to determine storage weight. Material of the remaining plants from each treatment replication was mixed, homogenized, and conjugated samples (3 samples per treatment) from mixed plant material were used for analysis. All analytical measurements were performed in three analytical replications.
Biometric measurements. Fresh (FW) and dry (DW) plant weights were measured using an electronic scale (Mettler Toledo AG64, Columbus, OH, USA) before and after tissue dehydration by freeze-drying (FD-7, SIA Cryogenic and Vacuum Systems, Venspils, Latvia). The plant leaf area was measured with an automatic leaf area meter (AT Delta-T Devices, Burwell, UK).
Derivative parameters were calculated from biometric measurement data. The shoot-to-root ratio was calculated from the dry weight of the plant above-ground part and the root dry weight. ΔFW/ΔPPFD index was calculated as the ratio between the difference in plant fresh above-ground part weight (%) and the increase (%) in lighting PPFD between adjacent lighting treatments [
30].
Transpiration rate (TR, g kg
−1 h
−1) [
31] was calculated as the alteration in weight of perilla plants over storage time:
where HW is the harvest fresh weight; SWt is the storage weight after selected storage duration; and t is the storage duration, measured in hours.
Weight loss (WL, %) was evaluated after storage, while water content (WC, %) [
32] was calculated both at harvest and after 1, 3, and 5 days of storage:
where HW is the harvest fresh weight; and SWt is the storage weight after selected storage duration.
where FW is the harvest or storage fresh weight; DW is the dry plant weight.
Light use efficiency (LUE, g mol
−1 m
−2) [
33] was expressed as dry mass production per square meter per total incident light:
where DW is the dry plant weight; N is the number of plants per square meter; DLI is the daily light integral per square meter; and T is the duration of the cultivation cycle, as measured in days.
For biochemical properties, antioxidant potential, sugar, protein, and chlorophyll contents in perilla plants were evaluated at harvest and after selected storage durations.
To evaluate the antioxidant properties of perilla plants, the extract was prepared by grinding 0.05 g of freeze-dried plant material and diluting it with 5 mL of 80% methanol. After 24 h of incubation, samples were centrifuged (Hermle Z300K, Baden-Württemberg, Germany) at 4500 rpm. The supernatant was used to measure DPPH (2-diphenyl-1-picrylhydrazyl) [
34] and ABTS (2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) [
35] free radical scavenging activities (Trolox equivalent free radical scavenging activity (mmol TE g
–1 DW)), as well as ferric reduction antioxidant power (FRAP; µmol of Fe
2+ reduced by g
−1 of dry plant weight (µmol g
–1 DW)) [
36] and the total content of phenolic compounds [
37], using previously described analytical procedures [
38] on Spectro-star Nano microplate reader (BMG Labtech, Ortenberg, Germany).
Soluble sugar contents were determined in an aqueous extract of freeze-dried plant material, using high-performance liquid chromatography (Nexera HPLC system, Shimadzu, Kyoto, Japan) analysis with an evaporative light scattering detector (ELSD LT IIShimadzu, Kyoto, Japan), using a previously described method [
39]. Fructose, glucose, and sucrose contents were expressed as mg of sugar per g of plant dry weight (DW).
Total protein contents were determined spectrophotometrically using the Bradford reagent (Sigma-Aldrich, Louis, MO, USA) and Bovine serum albumin (0.05–1.0 mg mL−1; Sigma-Aldrich, Louis, MO, USA) as an external standard. Ten μL of sample or standard were added to 190 μL of diluted Bradford reagent, and after the incubation period, absorption at 595 nm was measured using a Spectro-star Nano microplate reader (BMG Labtech, Ortenberg, Germany). Protein content was presented as mg per g of plant dry weight (DW).
Chlorophyll a, b, and β carotene contents were determined using the previously described HPLC method [
38] in 80% acetone extracts. Quantitative evaluation was performed according to calibration curves obtained by measuring external standard solutions. Pigment contents were presented as mg per g of plant dry weight (DW).
Statistical evaluation. All the experiments were performed in three replications, and each measurement was performed 3 times. The significant differences between means of measured parameters in perilla plants cultivated under different lighting intensity treatments were distinguished using one-way ANOVA, Tukey’s HSD test analysis at the confidence level of p ≤ 0.05. For result modeling, a principal component analysis (PCA) test was performed. For data processing, Microsoft® Excel® for Microsoft 365 MSO (Version 2410 Build 16.0.18129.20100) and compatible XLStat 2022.3.1 (Addinsoft, Paris, France) software tools were employed.
3. Results
Lighting PPFD range of 150–300 µmol m
−2s
−1, common for controlled environment cultivation, was selected for experiments. Results indicate that even 50 µmol m
−2s
−1 disparity in this range had a pronounced impact on
Perilla frutescens biomass productivity at harvest and its post-harvest quality. The PPFD of 300 µmol m
−2s
−1 (
Figure 2) resulted in the highest plant leaf area. Plant fresh and dry weight were twice as high at 300 µmol m
−2s
−1, compared to the lowest light treatment of 150 µmol m
−2s
−1. 200 µmol m
−2s
−1 lighting intensity resulted in a remarkably lower shoot-to-root ratio (
Figure 2c) than 150 µmol m
−2s
−1 lighting intensity treatment, indicating more pronounced biomass allocation to roots. Also, though there was no significant difference in light use efficiency between 250 and 300 µmol m
−2s
−1 PPFD treatments, the highest shoot-to-root ratio, as well as ΔFW/ΔPPFD index, were determined for perilla plants cultivated under 250 µmol m
−2s
−1 PPFD (
Figure 2b,c).
There is an equal 50 µmol m
−2s
−1 difference between all adjacent PPFD treatments. However, PPFD shifts from 150 to 200, 200 to 250, and 250 to 300 µmol m
−2s
−1 lead to 33%, 25%, and 20% increases in lighting intensity, respectively (
Figure 1). ΔFW/ΔPPFD index emphasizes the balance between plant biomass gain and increase in lighting PPFD percentage between adjacent treatments. Results show (
Figure 2c) that according to this index, the PPFD shift from 150 to 200 µmol m
−2s
−1 is not consequential regarding biomass gain. However, shifting the PPFD from 200 to 250 µmol m
−2s
−1 shows a pronounced economic benefit, as the 25% increase in lighting intensity within this range leads to the highest fresh weight gain. Further increases in lighting PPFD from 250 to 300 µmol m
−2s
−1 results in a 2.2 times lower ΔFW/ΔPPFD value.
Higher lighting PPFD also resulted in lower transpiration rates (TR) and, therefore, significantly lower weight loss (WL) (
Table 1). After 1 day of storage, WL and TR were determined to be about two times lower compared to lower cultivation PPFD treatment. However, the impact of cultivation lighting PPFD on derivative parameters is more pronounced after 1 day of storage, when after a longer 3 and 5-day period, the positive effect of higher lighting PPFD diminishes.
Biochemical parameters and preservation of internal quality during storage of
Perilla frutescens also depend on the cultivation lighting conditions. Soluble sugar (
Figure 3) contents differ between storage days more than between different PPFD treatments. In all treatments, the content of monosugar (fructose and glucose) increases, while sucrose is catabolized.
Antioxidant properties (
Figure 4) differences are also not pronounced at harvest in plants cultivated under different PPFD (except DPPH free radical scavenging activity) treatments; however, during storage, the highest investigated, 300 µmol m
−2s
−1 PPFD resulted in the better preservation of antioxidant properties (higher DPPH, ABTS free radical activity, total phenolic contents) after 1 and 3 days of post-harvest storage. For example, after 3 days of storage, DPPH free radical activity was determined to be 70 and 35% higher in perilla plants cultivated under 300, compared to 150 and 250 µmol m
−2s
−1, respectively.
The protein content at harvest, the level of decline in protein contents (
Table 2), and chlorophyll contents during storage are also not affected remarkably by lighting PPFD of 150–300 µmol m
−2s
−1.
4. Discussion
The promotion of plant growth under increasing DLI depends on the cohesion of PPFD and photoperiod [
4,
40].
Perilla frutescens is the obligatory short-day plant. Therefore, photoperiod studies have explored the induction of flowering [
41]. There has been relatively less research focus on the impact of light intensity on high-quality leaf and seedling production. Moreover, previous studies show that lighting PPFD effects are plant genotype-specific [
11,
30] and require individual optimization. Expanding the portfolio of plants cultivated in CEA knowledge of the performance of diverse leafy vegetables under different lighting intensities will be necessary to evaluate cultivation profitability. Moreover, it was published that different pre-harvest lighting strategies were efficient for improving and preserving the post-harvest nutritional quality of herbs and leafy greens, such as basil [
42,
43], lettuce [
20], kale, and pak choi [
21,
44].
In this study, the whole investigated PPFD interval of 150–300 µmol m
−2s
−1 was acceptable for expected perilla productivity and internal quality parameters. However, the highest accumulated dry weight at harvest, as well as nutritional quality parameters during 1–5 days of storage, were the best preserved in perilla plants cultivated under the highest investigated PPFD of 300 µmol m
−2s
−1. The cultivation lighting intensity had the most pronounced impact on evaluated parameters at harvest and after 1-day post-harvest storage, which was confirmed by the distribution of the variables according to the F2 component in the PCA scatterplot (
Figure 5) due to the variation in water content and chlorophyll B content. Further, after 3 and 5 days of storage, the level of the impact of cultivation lighting PPFD on measured productivity and quality parameters diminishes. Notwithstanding, according to F1 square cosines of the values, perilla plants, after 3–5 days of storage, significantly differ from those measured at harvest by fresh weight, antioxidant properties, sugar contents, and water loss parameters.
The shelf life of vegetables depends on post-harvest handling and a wide range of pre-harvest factors, including genetic and environmental parameters [
45]. Studies with butterhead and iceberg lettuces confirmed higher cultivation lighting intensity effects on improved shelf life [
46]. Min et al. 2021 [
20] reported that high light intensities applied shortly at the end-of-production lighting also increased the percentage of dry matter and contents of ascorbic acid and carbohydrates at harvest in loose-leaf lettuce, and these increased levels were maintained during post-harvest storage. Similar effects of the end-of-production lighting intensity on prolonged shelf-life and antioxidant contents and their preservation were reported in basil [
47], which belongs to the same
Lamiaceae family as perilla plants. Gudžinskaitė et al. 2024 [
38] also concluded that higher antioxidant contents in mustard and kale microgreens correspond to mild photo stress, created by higher pre-harvest lighting PPFD conditions, and it has a lasting effect during post-harvest storage. In agreement with the results of this study, the prolonged post-harvest shelf life and antioxidant status of plant material are based on the plant’s photosynthetic efficiency and antioxidant status at harvest. During post-harvest storage, vegetables often increase the production of reactive oxygen species (ROS), which depletes their antioxidant pools and diminishes their nutritional value [
48]. According to the results of this study, perilla leaf antioxidant properties at harvest are not proportional to the antioxidant properties after post-harvest storage. Different cultivation lighting PPFD resulted in unequal antioxidant activity depletion rates during 1 and 3 days of storage. A variety of reduction-oxidation reactions, along with both enzymatic components (such as superoxide dismutase, catalase, and ascorbate peroxidase) and non-enzymatic components (including vitamins, polyphenols, and carotenoids), play a role in balancing reactive oxygen species (ROS). Further in-depth analysis is needed to clarify the mechanisms involved and to substantiate the impact of cultivation lighting intensity on these processes.
Tailoring pre-harvest lighting intensity is a promising tool for nutritional value improvement, sugar, pigment, and antioxidant content preservation during post-harvest storage in the dark, in
Perilla frutescens and other leafy vegetables. However, other findings disclose that the pre-harvest lighting spectrum also had a significant impact on volatile, bioactive compounds, and antioxidant activity in basil [
35]. Therefore, further species- and cultivar-specific investigations in the context of other cultivation environment conditions are needed to determine the impact of light intensity and quality administration during cultivation shelf-life and quality extension.
Along with the plant physiological role of lighting intensity, economic aspects must be considered in real-world CEA scenarios with the objective of at least equating the cost of lighting with the financial benefit of sales [
49]. Transitioning from high-pressure sodium (HPS) to LED lighting resulted in ~40% energy savings for lighting [
50]. Still, in controlled environment facilities, electricity can account for 28% of operational costs [
51], with the lighting system as the major energy carrier (65 to 85%) [
52]. While photon efficacy is widely discussed, seeking to substantiate the economic efficiency of LED lighting [
53], the choice of PPFD cultivation lighting also significantly affects the initial lighting installation expenditures (CAPEX), as well as operational expenses (OPEX). The results of this study show that a 25% increase in lighting intensity from 200 to 250 µmol m
−2s
−1 resulted in 57% higher plant fresh weight, as well as a further 20% PPFD increment from 250 to 300 µmol m
−2s
−1, which also resulted in a 58% higher plant fresh weight. However, the ratio between the percental rise in PPFD and biomass gain ΔFW/ΔPPFD, suggests that shifting light from 200 to 250 µmol m
−2s
−1 is more economically beneficial than increasing it from 250 to 300 µmol m
−2s
−1. Notwithstanding, together with biomass productivity, plant quality and its post-harvest preservation provide additional values. Therefore, the question of whether the marginal gains in post-harvest quality will justify the increased energy costs of using higher lighting intensity levels, should be analyzed individually, including specific technological aspects of lighting units and the grower’s objectives.