1. Introduction
The global demand for reduced CO
2 emissions has increased the attention paid to the use of renewable energy, including green hydrogen as an energy vector. Energy storage is a key issue to be addressed for the widespread adoption of renewable energy in domestic or export markets [
1,
2,
3]. In this regard, liquid hydrogen is a likely medium for storing, transporting, and using renewable energy [
4] over a wide range of scales and applications [
5]. Liquefaction methods for hydrogen typically involve a combination of compression, expansion, and throttling processes, such as with the Linde–Hampson cycle [
5,
6]. Currently, on an industrial scale, the largest single liquefier has a capacity of 32 T/day [
5]. In these cases, cyclic gas compression techniques are the predominant cooling methods, and contribute to the high operational and capital costs of many installations [
5,
7].
Recent attention has been directed at magnetic refrigeration (MR) methods, with a focus on small- to modest-scale refrigeration techniques, as succinctly described in a review article by Kitanovski [
8]. Kitanovski [
8] suggests that the low Carnot efficiency of existing refrigeration at a small scale offers substantial room for improvement by using MR technologies. An exemplar use of MR is described by Archipley et al. [
9], who use active magnetic regenerative refrigeration (AMRR) to liquefy methane at room temperature.
Magnetocaloric (MC) materials show properties that invoke an isothermal magnetic entropy change or an adiabatic temperature change with the application or removal of an external magnetic field. This phenomenon is known as a magnetocaloric effect (MCE), with key applications in MR. The use of MCE has been demonstrated for room temperature applications with compounds such as Gd
5Si
2Ge
2, (Mn,Fe)
2P, La(Fe,Si)
13H, and Gd
0.8875Ce
0.1025Si
0.84Cr
0.19 [
10,
11,
12,
13,
14]. MR research has also focused on cryogenic temperatures [
15,
16,
17,
18,
19], especially for hydrogen liquefaction, which occurs at 20 K at atmospheric pressure. A report by Ihnfeldt et al. [
20] shows that an MR system that achieves 50% Carnot efficiency in the 20–80K region would provide an 85% reduction in electrical costs and a 60% reduction in the capital equipment cost compared to traditional compression-based cryocoolers.
Bykov et al. [
17] and Tang et al. [
19] have shown that combinations of stacked MC materials are suitable for cooling from 77 K to liquid hydrogen temperature. Material combinations include first-order (FOPT) and second-order phase transition (SOPT) MCE compounds such as ErCo
2 and HoB
2 [
19], or tuning the Curie temperature, T
C, with various substituent elements (e.g., Ho
1−xDy
xAl
2 or Ho
1−xGd
xB
2) [
17,
21]. A giant MCE reported for the rare-earth diboride, HoB
2, shows strong potential for low-temperature applications at or near the Curie temperature, T
C, of ~15 K [
22]. The maximum change in magnetic entropy, ΔS
M, for HoB
2 at 5 T is 40.1 Jkg
−1K
−1 [
22], and is of optimum practical use near 15 K. Other Ho-based compounds such as HoAl
2 and Ho
1-xGd
xB
2 show maximum ΔS
M values above and below 20 K at 5 T [
17,
21].
In this work, we consider combinations of Ho-based compounds that may be suitable for MR at temperatures <77 K. For hydrogen liquefaction plants on an industrial scale, superconducting magnets are considered a highly effective choice [
17,
18], not only for large-scale production [
17], but also because fields of 10 T or higher are attainable. We explore applied magnetic fields up to 10 T combined with an increase in T
C via compatible element substitution into HoB
2. In this study, we report on Nb substitution in the Ho-B alloy system and the effects on the microstructure and magnetocaloric properties.
2. Materials and Methods
Polycrystalline samples of Ho-B compounds are prepared on a water-cooled copper hearth via arc melting using a tungsten electrode, and high-purity Ar. Stochiometric amounts of Ho (95% purity, supplied by Sigma Aldrich, Ryde, NSW, Australia) and nano boron powder (99.8% purity, supplied by Pavezynum Co., Gebze, Kocaeli, Turkey) are weighed and pressed into a pellet of a weight of 2 g. Details of the impurities detected in the Ho powder using ICP-OES analysis are provided in
Supplementary Materials Table S1. For substituted samples, molar ratios of Ho, B, and Nb are also weighed (for nominal 10% of a substituent element), mixed, and pressed into a pellet of a weight of 2 g. Both pellets are formed under an applied pressure of 10 tonnes for 2 min.
The pellets are then melted in an arc furnace on a water-cooled copper hearth under Ar. The use of a water-cooled hearth with the arc melting technique helps to reduce contamination from the crucible that contains the starting mixture, and allows for the easy removal of oxygen from the surrounding gas in the arc furnace. Prior to arc melting experiments, the chamber is vented and filled with Ar three times, and then re-filled with Ar in order to eliminate oxygen in the chamber. The Ar atmosphere is further purified using Ti foam before melting each pellet. In order to ensure element homogeneity in the mixed material, the ingot is turned and remelted four times utilizing the same heating and cooling rates for each sample.
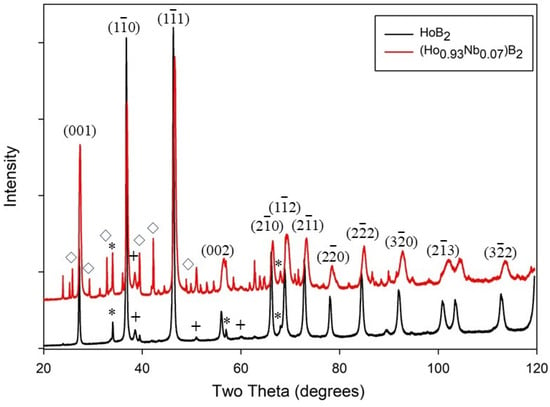
The microstructural and compositional analyses were performed using standard metallographic practices on polished samples mounted in conductive resin. The crystal structure and phase identification were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), and EDS microanalysis using secondary X-rays. The XRD patterns of samples were measured using Co Kα1 radiation in Bragg–Brentano geometry, with 2θ steps of 0.02° and a counting time of 10 s per step, utilizing D8 Bruker X-ray diffractometers (Bruker, Billerica, MA, USA). The diffraction patterns are refined and indexed using the software programme Topas [
23]. Detailed analyses using XRD patterns and SEM+EDS indicate that all synthesized samples are multiphase, with HoB
2 being the predominant phase.
Electron Backscatter Diffraction (EBSD) data were obtained using a field emission scanning electron microscope (FESEM, JEOL 7001 SEM, Japan Electron Optics Ltd., Tokyo, Japan) with automated feature detection and equipped with an SDD XMax 50 mm2 detector, pattern analyzer, and Channel 5 analysis software (Oxford Instruments plc, Abingdon, Oxfordshire, UK). EBSD mapping was conducted at an accelerating voltage of 20 kV and a step size of 0.2 µm.
The temperature and field dependence of dc magnetization measurements were calculated using a Dynacool Physical Property Measurement System (PPMS) with a Vibrating Sample Magnetometer (VSM) from Quantum Design (San Diego, CA USA), in the temperature range of 5–71 K and a dc magnetic field from 0 T to 10 T. The temperature dependence of magnetization (M(T)) was measured under the zero field cooled (ZFC) and field cooled (FC) protocols. The magnetic entropy change is calculated from the isothermal field dependent magnetization curves using Maxwell’s relation [
24]:
where Hi is the initial magnetic field and Hf is the final magnetic field.
4. Discussion
Liquefaction is important for gas storage and transportation, as is evident for the natural gas industry [
35] and for specific existing uses of hydrogen [
5]. The broadened utilization of liquid hydrogen for “hard-to-abate” industry sectors [
36], as well as for transportation [
36], may be rapidly effected by more compact and efficient technologies such as MR [
8]. Continuous cooling using magnetocaloric materials requires both rapid variation in magnetic fields [
37] and the use of magnetic material as a regenerator in an Active Magnetic Regenerative Refrigerator (AMRR) [
8,
18]. A large entropy change for magnetocaloric materials is preferably obtained at magnetic fields above 2 T [
16], most effectively deployed with superconducting magnets [
37]. In general, the value for magnetic enthalpy, |ΔS
M|, increases with an increased applied magnetic field [
38], as shown in
Figure 5a for both Ho diboride compounds up to 10 T and for other Ho compounds up to 5 T [
21,
22,
32].
As shown by de Castro et al., [
22] the magnetic entropy change for HoB
2 is at a maximum value of 40.1 J. kg
−1K
−1 near the Curie temperature (T
C~15 K) for a magnetic field change of 5 T [
19,
38,
39,
40,
41]. Because the Curie temperature for HoB
2 is close to the temperature for liquid hydrogen (20.3 K), this compound is a promising candidate for AMRRs [
22,
38]. However, the maximum change in entropy, ΔS
M, for HoB
2, decreases with the increasing temperature for the same applied field, and so is of optimum practical use at, or near, 15 K. Hence, a range of elements have been substituted into HoB
2 [
21,
32,
39] in order to either (i) increase the T
C and/or to (ii) increase ΔS
M across a broad temperature range.
Iwasaki et al. [
39] show that, in the absence of a solid solution between end-member alloys (e.g., HoB
2 and HoAl
2), the MCE is only due to that of the dominant magnetocaloric material. As a result, it is important to establish the solubility limit(s) of potential substituents of HoB
2 or similar alloys. For other substituted alloys, such as Ho
1−xDy
xB
2 [
32], T
C increases with increased Dy substitution up to x = 1 for an applied field of 5 T. However, with increased T
C, the magnitude of |ΔS
M| decreases, although the temperature range of the |ΔS
M| curve increases [
32]. Similarly, for Ho
1-xGd
xB
2 alloys (for 0 < x < 0.4), an applied field of 5 T results in an increase in T
C and broadens the |ΔS
M| curve(s) without the magnetic hysteresis effects of the Dy analogue [
21]. For the Gd-substituted series, the T
C increased to between 17 K and 30 K [
21]. This increase in T
C offers the potential to deliver a relatively high refrigerant capacity across a wide temperature range (e.g., from 15 K to 30 K or higher) with HoB
2 and appropriate stoichiometric substitutions of soluble elements.
The phase field for Ho with B shows that HoB
2 forms at a peritectic temperature of 2200 °C due to the decomposition of solid HoB
4 and a Ho-rich liquid, with complete formation at 2350 °C [
42]. Noting the relatively low purity of Ho starting material at 95% (
Supplemental Table S1), we suggest that the presence of impurities in the final product may be reduced with higher quality Ho. We have considered the potential for impurity phases, such as Ho
2O
3 and HoB
4, to affect the magnetocaloric properties of HoB
2 and Nb-substituted HoB
2. For HoB
4, the presence of antiferromagnetic transitions at 7.1 K and 5.7 K [
43,
44] are a proxy indicator for effect(s) on the magnetocaloric properties of HoB
2 samples. Similarly, an antiferromagnetic transition occurs for amorphous and crystalline Ho
2O
3 at 2.1 K and 5.2 K, respectively [
45,
46]. As shown in
Figure 3a,b, such anomalies are not observed for HoB
2 and Ho
0.93Nb
0.
07B
2. Therefore, we suggest that the minor amounts of HoB
4 and Ho
2O
3 have a limited or no effect on the magnetic transition temperature of these arc-melted samples.
Both Ho-boride samples show a pronounced peak at or near the Curie temperature, T
C. The T
C increase to 17.5 K for Ho
0.93Nb
0.07B
2 is due to successful substitution of Nb into the HoB
2 structure. A second magnetic transition marked by T
* at 11 K is observed for both HoB
2 and Nb-substituted HoB
2. The origin of T
* is likely due to a spin reorientation mechanism, as identified for Dy- and Gd-substituted HoB
2 [
21,
32]. The addition of a spherical S
7/2 Gd
3+ moment in HoB
2 induces an enhancement in the Curie temperature, a reduction in the peak value of ΔS
M, and a broadening of the ΔS
M curves [
21]. The values for Ho
0.93Nb
0.
07B
2 show a similar trend, with an increase in T
C and reduction of ΔS
M for all applied fields up to 10 T. We suggest that the increased substitution of Nb into HoB
2 will further increase T
C, with a consequent increase in the temperature range at which ΔS
M is viable for effective MR.
The relative reduction in RCP for Ho
0.93Nb
0.
07B
2 compared with HoB
2 can also be attributed to the substitution of Nb for Ho, which alters magnetic interactions and the structural ordering within the material. Nb substitution weakens the overall magnetic moment and decreases MCE efficiency, thereby reducing the RCP. Despite the lower RCP, the linear trend in
Figure 5b suggests that both materials maintain a predictable response to increasing fields, suggesting that Nb-substituted HoB
2 compounds are candidates for further exploration in field-dependent cooling applications.
Table 3 summarizes the magnetocaloric properties of HoB
2 and Ho
0.93Nb
0.07B
2 from this study, as well as for other SOPT Ho compounds [
8,
21]. The maximum ΔS
M value for HoB
2 in this work under an applied field of 5 T (34.3 Jkg
−1K
−1) is comparable with the value of 40.1 Jkg
−1K
−1 for arc-melted samples reported by de Castro et al. [
22]. The ΔS
M value reported for gas-atomized particles of HoB
2 near the T
C of 15 K is also 40.1 Jkg
−1K
−1 [
47], and slightly lower in the data reported by Yamomoto et al. [
41]. In these cases, XRD data show that the HoB
2 samples contain minimal or no impurities [
22,
41,
47], unlike those noted in
Table 1 for HoB
2 in this work and for Ho
0.93Nb
0.
07B
2.
For HoB
2 produced in this study and shown in
Table 1, impurities account for ~9% of the final ingot and thus, a lower weight fraction of HoB
2. Similarly, for Ho
0.93Nb
0.
07B
2, the reduction in weight fraction of other Ho compounds is ~25%. Adjusting the weight-dependent values for both samples suggests that at an applied field of 5 T, the maximum ΔS
M for HoB
2 and Ho
0.93Nb
0.
07B
2 from this study would be ~38 Jkg
−1K
−1 and ~35 Jkg
−1K
−1, respectively. This approach is consistent with estimates of ΔS
M by Iwasaki et al. [
39] when evaluating the impact of Al substitution into HoB
2. For an applied field of 10 T, the weight-adjusted values of a maximum ΔS
M are ~52 Jkg
−1K
−1 and ~49 Jkg
−1K
−1 for HoB
2 and Ho
0.93Nb
0.
07B
2, respectively.
For both examples of HoB
2 in
Table 3, the RCP values are similar at 5 T and suggest that marginal differences in magnetic parameters (e.g., T
C and ΔS
M) and/or a modest level of impurities may enable a comparable refrigeration capacity in an operating system. In practice, the data in
Table 1 and
Table 3 imply that low levels of impurities are unlikely to substantially affect ΔS
M at high applied fields (i.e., > 2 T). This implication is consistent with a detailed study on the presence of non-stoichiometric phases formed by inductive melting gas atomization [
41]. The work by Yamomoto et al. [
41] showed that the presence of up to 20 wt. % impurity phases has minimal effect on the physical properties of HoB
2 particles, while retaining a value for ΔS
M well above 30 Jkg
−1K
−1 at 5 T. By comparing the maximum values of ΔS
M for HoB
2 at 5 T from earlier works [
22,
30,
41,
47] and this study, we estimate an average value of 39.1 (±1.5) Jkg
−1K
−1, suggesting that sample preparation for HoB
2 may have a limited influence on magnetic properties. Nevertheless, for HoNi synthesis, Rajivghandi et al. [
48] have shown a difference of 8 K in the T
C values between melt-spun and arc-melted samples. The values for HoNi in
Table 3 are for an arc-melted sample [
48].
The identification of Nb solubility in HoB
2 suggests that higher proportions of Nb, or other Group 5 elements, may enable a family of Ho
1-xM
xB
2 compounds (where M = Gd, Nb) suited to hydrogen liquefaction. With a combination of SOPT compounds, as noted above, or of similar analogues, an AMRR system based only on Ho compounds may be a realizable goal. SOPT magnetic materials, which include the compounds listed in
Table 3, and now including Ho
0.93Nb
0.07B
2,
, are without thermal hysteresis, and offer the capacity for reversibility and mechanical stability in a system undergoing cyclic performance [
19]. While temperatures from 77 to 20 K are not completely covered by the combination of compounds in
Table 3, there is potential to extend the T
C range from 15 to 36 K. The use of Ho compounds with additional element substitutions (e.g., HoCo
1.8Ni
0.15Al
0.05), as exemplified by Tang et al. [
19], and/or including Group 5 elements, show promise.
Superconducting magnets are necessary to achieve and maintain a higher field strength compared to magnetic field(s) from permanent magnets. This distinction is crucial for some practical applications, particularly for MR at cryogenic temperatures below 113 K [
5], and as shown in this and previous works [
17,
19,
21,
22]. The reduction in ∆S
M with Nb substitution in HoB
2 suggests a trade-off between increasing the T
C and maintaining a high magnetocaloric effect, as noted by others [
21].
Table 3 shows that for SOPT polycrystalline Ho compounds, both the ∆S
M and RCP values increase with an increased applied field up to 10 T. These data also suggest that despite a drop in the ΔS
M value, with higher T
C for a particular compound, the range of values for δT
FWHM available at higher magnetic fields (i.e., >5 T) provides an effective RCP using SOPT Ho-based magnetocaloric materials.