3.2.1. Synthesis of the Complexes Bearing N Donors (Complexes 1 and 2)
Complexes 1–2 were prepared using a similar procedure. More specifically, a methanolic solution (10 mL) containing a salt of the corresponding NSAID (0.4 mmol, either used as purchased or formed in situ by the addition of KOH into a solution of the NSAID) was added into a methanolic solution (~10 mL) of MnCl2·6H2O (0.2 mmol, 39 mg) followed by the addition of 2 mL of pyridine. The resultant solution was stirred for an hour and was left for slow evaporation at room temperature.
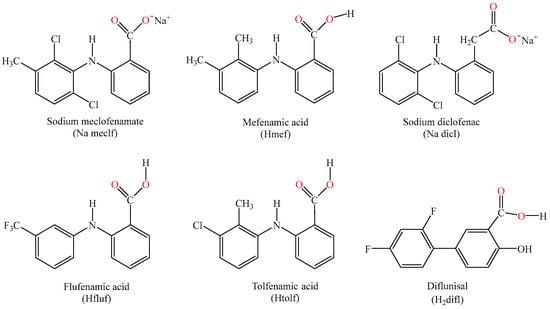
[Μn(meclf–O)2(py)2(H2O)2]∙2py (complex 1): Na meclf (0.4 mmol, 127 mg) was used as the salt of the NSAID. Colorless single crystals of complex 1 suitable for X-ray crystallography were isolated after 20 days. Yield: 90 mg, 45%. Anal. calcd for [Μn(meclf)2(py)2(H2O)2]∙2py (C48H44Cl4MnN6O6, MW = 997.65): C 57.79, H 4.45, N 8.42; found: C 57.61, H 4.35, N 8.30%. IR (KBr disk), vmax/cm−1: vasym(COO): 1579 (s); vsym(COO): 1385 (m); Δv(COO) = 194; ρ(C–H)py = 699 (s). UV-vis: as nujol mull, λ/nm: 303; in DMSO solution, λ/nm (ε/M−1cm−1): 306 (3100). The complex is soluble in DMSO and DMF and is non-electrolyte (ΛM = 9 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(mef–O)2(py)2(H2O)(MeOH)]∙1.5py (complex 2): KOH (0.4 mmol, 0.4 mL of 1 M solution) and Hmef (0.4 mmol, 97 mg) were used for the formation of salt of the NSAID. Colorless single crystals of complex 2 suitable for X-ray crystallography were isolated after 3 weeks. Yield: 75 mg, 45%. Anal. calcd for [Μn(mef)2(py)2(H2O)(MeOH)]∙1.5py (C48.50H51.50MnN5.50O6, MW = 862.40): C 67.55, H 6.02, N 8.93; found: C 67.35, H 5.85, N 8.70%. IR (KBr disk), vmax/cm−1: vasym(COO): 1581 (s); vsym(COO): 1389 (s); Δv(COO) = 192; ρ(C–H)py = 701 (s). UV-vis: as nujol mull, λ/nm: 331, 302; in DMSO solution, λ/nm (ε/M−1cm−1): 338 (5100), 306 (12500). The complex is soluble in DMSO and DMF and is non-electrolyte (ΛM = 8 mho∙cm2∙mol–1 in 1 mM DMSO).
3.2.2. Synthesis of the Complexes Bearing N,N’–Donors (Complexes 3–9)
Complexes 3–9 were prepared following a similar procedure. More specifically, a methanolic solution (5–10 mL) containing a salt of the corresponding NSAID (0.4 mmol, either used as purchased or generated in situ by the addition of KOH into a solution of the NSAID) was added into a methanolic solution (∼10 mL) of MnCl2·6H2O (0.2 mmol, 39 mg) followed by the addition of the corresponding N,N’–donor (0.2 mmol) (i.e., neoc, phen, bipy). After stirring for 1 h, the reaction solution was left to evaporate slowly at room temperature.
[Μn(meclf–O)2(phen)(MeOH)2] (complex 3): Na meclf (0.4 mmol, 127 mg) was used as the NSAID salt, and phen (0.2 mmol, 36 mg) was the corresponding N,N’–donor. Yellow single crystals of complex 3 suitable for X-ray crystallography were isolated after 24 h. Yield: 125 mg, 70%. Anal. calcd for [Μn(meclf)2(phen)(MeOH)2] (C42H36Cl4MnN4O6, MW = 889.51): C 56.71, H 4.08, N 6.30; found: C 56.88, H 3.95, N 6.18%. IR (KBr disk), vmax/cm−1: vasym(COO): 1579 (s); vsym(COO): 1382 (m); Δv(COO) = 197; ρ(C–H)phen = 729 (s). UV-vis: as nujol mull, λ/nm: 307; in DMSO solution, λ/nm (ε/M−1cm−1): 310 (5700). The complex is soluble in DMSO, and DMF and is non-electrolyte (ΛM = 7 mho∙cm2∙mol–1 in 1 mM DMSO).
[Mn(meclf–O)2(bipy)(MeOH)2] (complex 4): Na meclf (0.4 mmol, 127 mg) was used as the salt of the NSAID and bipy (0.2 mmol, 31 mg) was the corresponding N,N’–donor. Yellowish single crystals of [Mn(meclf)2(bipy)(MeOH)2] suitable for X-ray structure determination were deposited after 2 weeks. Yield: 110 mg, 64%. Anal. calcd for [Mn(meclo)2(bipy)(MeOH)2] (C40H36Cl4MnN4O6, MW = 865.49): C 55.51, H 4.19, N 6.47; found: C 55.67, H 4.03, N 6.29%. IR (KBr disk), vmax/cm−1: vasym(COO): 1580 (s); vsym(COO): 1383 (s); Δv(COO) = 197; ρ(C–H)bipy = 761 (s). UV-vis: as nujol mull, λ/nm: 315, 293; in DMSO solution, λ/nm (ε/M−1cm−1): 319 (16000), 287 (25000). The complex is soluble in DMSO and DMF and is non-electrolyte (ΛM = 6 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(dicl–O,O’)2(neoc)]∙0.25 H2O (complex 5): Na dicl (0.4 mmol, 92 mg) was used as the NSAID salt and neoc (0.2 mmol, 46 mg) was the corresponding N,N’–donor. Yellow single crystals, suitable for X-ray structure determination, were collected after 2 days. Yield: 105 mg, 60%. Anal. calcd for [Μn(dicl)2(neoc)]∙0.25 H2O (C42H32.5Cl4MnN4O4.25, MW = 857.98): C 58.80, H 3.82, N 6.53; found: C 58.70, H 3.75, N 6.67%. IR (KBr disk), vmax/cm−1: vasym(COO): 1590 (s); vsym(COO): 1415 (s); Δv(COO) = 175; ρ(C–H)neoc = 731 (m). UV-vis: as nujol mull, λ/nm: 370, 289, 275; in DMSO solution, λ/nm (ε/M−1cm−1): 368 (1700), 285 (13300), 271(14400). The complex is soluble in DMSO and is non-electrolyte (ΛM = 8 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(mef–O,O’)2(neoc)]∙1.5MeOH∙0.25 H2O (complex 6): KOH (0.4 mmol, 0.4 mL of 1 M solution) and Hmef (0.4 mmol, 97 mg) were used for the formation of NSAID salt and neoc (0.2 mmol, 46 mg) was the corresponding N,N’–donor. Pale-yellow single crystals of complex 6 suitable for X-ray crystallography were isolated after 2 days. Yield: 70 mg, 45%. Anal. calcd for [Μn(mef)2(neoc)]∙1.5MeOH∙0.25 H2O (C45.5H46.5MnN4O5.75, MW = 796.32): C 68.63, H 5.89, N 7.04; found: C 68.50, H 5.75, N 6.81%. IR (KBr disk), vmax/cm−1: vasym(COO): 1582 (s); vsym(COO): 1396 (s); Δv(COO) = 186; ρ(C–H)neoc = 731 (s). UV-vis: as nujol mull, λ/nm: 334, 290, 275; in DMSO solution, λ/nm (ε/M−1cm−1): 339(sh) (3200), 291 (11800), 272 (11700). The complex is soluble in DMSO and is non-electrolyte (ΛM = 9 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(fluf–O,O’)2(neoc)] (complex 7): KOH (0.4 mmol, 0.4 mL of 1 M solution) and Hfluf (0.4 mmol, 112 mg) were used for the formation of NSAID salt and neoc (0.2 mmol, 46 mg) was the corresponding N,N’–donor. Yellowish single crystals of complex 7, suitable for X-ray crystallography, were collected after one month. Yield: 115 mg, 70%. Anal. calcd for [Μn(fluf)2(neoc)] (C42H30F6MnN4O4, MW = 823.64). C 61.25, H 3.67, N 6.80; found: C 61.40, H 3.55, N 6.68%. IR (KBr disk), vmax/cm−1: vasym(COO): 1582 (s); vsym(COO): 1397 (vs); Δv(COO) = 185; ρ(C–H)neoc = 729 (s). UV-vis: as nujol mull, λ/nm: 279; in DMSO solution, λ/nm (ε/M−1cm−1): 275 (15300). The complex is soluble in DMSO and is non-electrolyte (ΛM = 10 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(tolf–O,O’)2(neoc)] (complex 8): KOH (0.4 mmol, 0.4 mL of 1 M solution) and Htolf (0.4 mmol, 104 mg) were used for the formation of NSAID salt and neoc (0.2 mmol, 46 mg) was the corresponding N,N’ donor. Yellow single crystals of [Μn(tolf–O,O’)2(neoc)], 8 suitable for X-ray structure determination were deposited after 2 days. Yield: 100 mg, 64%. Anal. calcd for [Μn(tolf)2(neoc)] (C42H34Cl2MnN4O4, MW = 784.59): C 64.30, H 4.37, N 7.14; found: C 64.18, H 4.25, N 7.27%. IR (KBr disk), vmax/cm−1: vasym(COO): 1580 (s); vsym(COO): 1390 (vs); Δv(COO) = 190; ρ(C–H)neoc = 730 (m). UV-vis: as nujol mull, λ/nm: 300, 279; in DMSO solution, λ/nm (ε/M−1cm−1): 298 (22900), 272 (21600). The complex is soluble in DMSO and is non-electrolyte (ΛM = 9 mho∙cm2∙mol–1 in 1 mM DMSO).
[Μn(Hdifl–O,O’)(Hdifl–O)(neoc)]·0.5MeOH (complex 9): KOH (0.4 mmol, 0.4 mL of 1 M solution) and H2difl (0.4 mmol, 100 mg) were used for the formation of NSAID salt and neoc (0.2 mmol, 46 mg) was the corresponding N,N’–donor. Yellow single crystals of complex 9 suitable for X-ray crystallography were collected after 10 days. Yield: 75 mg, 48%. Anal. calcd for [Μn(Hdifl)2(neoc)]·0.5MeOH (C40.50H28F4MnN2O6.50, MW = 777.58). C 62.56, H 3.63, N 3.60; found: C 62.75, H 3.50, N 3.65%. IR (KBr disk), vmax/cm−1: vasym(COO): 1595 (m); vsym(COO): 1413 (m); 1379 (m); Δv(COO) = 182, 216; ρ(C–H)neoc = 732 (m). UV-vis: as nujol mull, λ/nm: 273; in DMSO solution, λ/nm (ε/M−1cm−1): 270 (17100). The complex is soluble in DMSO and is non-electrolyte (ΛM = 7 mho∙cm2∙mol–1 in 1 mM DMSO).
Source link
Filitsa Dimiza www.mdpi.com