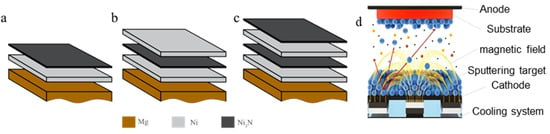
3.1. Structure Characterization of Composite Film
Figure 2 presents the X-ray diffraction (XRD) patterns for the composite film samples featuring three distinct layer structures. Prominent diffraction peaks were observed at 2θ values of 44.5°, 52.3°, and 76.2° in all three samples. Compared to the standard Powder Diffraction File (PDF) card of Ni, which corresponds to the Ni (1 1 1), Ni (2 0 0), and Ni (2 2 0) crystal faces, it is evident that the film layer predominantly consists of Ni, with a preferred orientation along the (2 2 0) plane. In addition to the Ni peaks, the diffraction pattern also displays peaks at 38.9°, 41.5°, and 44.5° and strong diffraction peaks at 41.5°. These correspond to Ni
3N (1 1 0), Ni
3N (0 0 2), and Ni
3N (1 1 1) crystal faces according to the PDF standard card for Ni
3N. This observation confirms the presence of Ni
3N in the film layer the crystallization is selected along (0 0 2), indicating that the composite film is indeed composed of both the Ni and Ni
3N phases. The Debye–Scherrer formula [
24] was used to estimate the grain sizes of Ni in SLF, ILF, and DLF composite films, which are 10, 7, and 6nm, respectively. With the increase in recombination times, the diffraction peak height of Ni
3N decreases first and then increases. The reason is that the ILF composite film deposited two layers of Ni continuously, resulting in an increase in the relative content of Ni in the composite film, so the diffraction peak intensity of Ni
3N decreased. However, due to the small-angle diffraction mode, the X-ray penetration depth is shallow, mainly reflecting the Ni near the surface and all Ni
3N, so the Ni
3N peak intensity is high in DLF.
The surface chemical states of the three composite films were determined by X-ray photoelectron spectroscopy (XPS).
Figure 3a,d,g show the measured spectra of ILF, SLF, and DLF, consisting mainly of Ni, C, N, and O, with the position of each peak calibrated against the position of C1s (284.8 eV).
Figure 3b,e,h show the XPS Ni2p spectra of the three composite films at high resolution. The peaks from Ni
3N/Ni are located at 852.9 eV, belonging to the Ni-Ni bond Ni 2p
3/2 (metal Ni), and the peaks from the Ni-N bond Ni 2p
3/2 are located at 853.7. Correspondingly, the peak of Ni
3N/Ni at 869.9 eV is Ni 2P
1/2 of the Ni-N bond [
25,
26]. Similarly, the N1s peaks are detected at a binding energy of 397.8 eV (as depicted in
Figure 3c,f,i). These peaks are characteristic of the Ni-N bond, and the correlation of these XPS findings with the XRD results strengthens the identification of the nickel nitride phase as Ni
3N in the composite film. The presence of both the O1s and NiO peaks at the binding energies of 855.5 eV for Ni2p
3/2 and 873.3 eV for Ni2p
1/2 in the XPS spectrum suggests that the surface nickel has been oxidized, resulting in the formation of nickel oxide [
27].
The cross-section morphology of the three laminated composite films is shown in
Figure 4. There is a clear boundary between the Ni and the Ni
3N layers, and the inter-layer bonding is tight. The EDS distribution results of elements also indicate that the composite film has a laminated structural nickel and nickel nitride consistent with the design. Prolonged deposition time has resulted in a strong columnar grain structure in the nickel layer. With its sufficient thickness, it serves as the main component of the composite film, playing a role in dissipating external forces. The Ni
3N layer, while relatively thin, is adequate to act as a supporting structure. The respective thicknesses of the two layers are 0.792 μm for the nickel layer and 0.191 μm for the Ni
3N layer, respectively.
The atomic force microscope observation results of the three-dimensional surface morphology of the composite film with three-layered structures are shown in
Figure 5.
Figure 5b shows that the surface morphology of Ni/Ni
3N/Ni (ILF) composite film is smoother and denser with a more uniform particle size distribution than that of the single and double laminated Ni/Ni
3N composite films. This is because nickel was deposited on the surface layer of Ni/Ni
3N/Ni composite film for 20 min, and, thus, nickel had sufficient time for lateral expansion while growing along the longitudinal column, so this coating presents a smooth and dense morphology This is because nickel was deposited on the surface layer of Ni/Ni
3N/Ni composite film for 20 min, and, thus, nickel had sufficient time for lateral expansion while growing along the longitudinal column, so this coating presents a smooth and dense morphology. On the other hand, single–double laminated Ni/Ni
3N composite film is a continuous deposition of Ni
3N layer on the surface of the Ni layer, and the lattice of nickel and nickel nitride is relatively similar. According to the growth mechanism of thin nickel nitride film on nickel surface described by the Stranski–Krastanov (S-K) model [
28], due to the strong interaction between the nickel layer and the deposited atoms of nickel nitride, the coating initially grows in layers, but with the increase in the thickness of the nickel nitride layer, the lattice mismatch increases, and the strain energy in the composite film gradually accumulates. When the composite film reaches a certain threshold, the composite film gradually tends to grow like an island and release the stress. In
Figure 5a,c, the white bright color is raised. The surface roughness results of the composite films with three kinds of laminated structures are shown in
Table 1.
3.2. Friction and Wear Properties of Composite Film
Table 2 shows the measurement results obtained for the wear volume and friction coefficient of the three composite films and AZ31 magnesium alloy. Compared to the magnesium alloy, the samples exhibit a substantial enhancement in wear resistance following the deposition of the composite film. Among them, the wear volume of the double-layered Ni/Ni
3N composite film (DLF) is significantly reduced, being almost one-tenth of the wear volume of the magnesium alloy sample. On the other hand, while the friction coefficient of various laminated composite films is slightly lower than that of magnesium alloys, the Ni/Ni
3N/Ni (ILF) films present a modestly elevated friction coefficient.
Figure 6 depicts the microscopic morphology of wear marks on several samples. The wear mechanism of the AZ31 magnesium alloy and its three composite films is primarily marked by adhesive wear. However, in the wear tracks left on the three composite films, the presence of fractured Ni
3N is distinctly observable. The analysis reveals that while both the AZ31 magnesium alloy and the composite film are characterized as polyphasic materials, the latter predominantly consists of nickel, which is distinguished by its high ductility. Moreover, it contains highly hard Ni3N, which, when experiencing external loads, reduces the local plastic deformation of the material and the tearing of the adhesive point due to the support that it provides, as well as the slow release and adjustment of the stress through deformation. Thus, the composite film possesses a high anti-wear ability. Increasing the number of layers layering in the composite film is an effective technique for significantly enhancing its wear resistance. The reduction in the friction coefficient is also related to the presence of highly hard Ni
3N on the surface of the film.
Table 3 presents the nano-hardness and Young’s modulus data obtained for the AZ31 magnesium alloy and three types of composite films. The nanoindentation hardness, Young’s modulus and adhesion strength of the composite film are significantly higher than those of the AZ31 magnesium alloy. This is because nickel has higher nano-hardness and elastic modulus than magnesium, and the Ni
3N layer in the composite film is directly related to the high hardness and rigidity of the latter; this evidences the strengthening effect of Ni
3N on the composite film and the adhesion strength decreases as the number of laminated layers increases. The Young’s modulus of the composite layer does not show a corresponding relationship with the position and number of the Ni
3N layers in the composite film, indicating that deposition of the Ni
3N layer has little influence on the Young’s modulus of the overall composite film.
Table 3 lists the hardness H and elastic modulus E, as well as the H/E values. The plastic deformation resistance coefficient H
3/E
2 [
29] was calculated as a measure of the wear resistance of the material. Compared with the wear data,
Table 3 shows that the “soft” Ni layer present at the surface of the composite film reduces its wear resistance. With the superposition of layers, the plastic deformation resistance of the composite film first decreases and then increases. The results show that the higher hardness of the Ni
3N layer formed at the surface of the composite film can improve the plastic deformation resistance of the latter, enhancing the wear resistance of the matrix.
Figure 7 shows the comparison of the Ni/Ni
3N film presented in this study with the literature for various nitride coatings and tool steels.
Figure 7 shows the hardness and wear coefficient of Ni/Ni
3N composite film compared with other coatings in this study. It is found that the hardness of Ni/Ni
3N composite film is between that of metal and nitride coating, but its wear coefficient is not much different from that of nitride film with high hardness. And, the plastic deformation resistance of the composite film is higher, so it has better toughness and can better resist external impact.
From a materials science perspective, Ni
3N, being a nitride of nickel, boasts excellent mechanical properties due to the strong d-sp electron orbital hybridization that occurs when nitrogen atoms are embedded into the Ni lattice [
34,
35,
36,
37]. When incorporated as the hard phase in a composite film, Ni
3N significantly boosts the overall hardness. Under external forces, the Ni layer absorbs and dissipates impact through plastic deformation, while the Ni
3N layer, owing to its high hardness, enhances both the hardness and the fracture resistance of the Ni/Ni
3N composite film, thereby improving its wear resistance. The Ni
3N layer in the Ni/Ni
3N composite film is introduced to enhance the film’s microstructure. Meanwhile, the Ni layer, acting as a middle layer, forms two strong interfaces with the substrate and the Ni
3N layer, which helps to alleviate the shear stress within the composite film and subsequently improves the bonding force between the film and the substrate. The Ni
3N layer in the Ni/Ni
3N composite film is introduced to enhance the film’s microstructure; meanwhile, the Ni layer, acting as a middle layer, forms two strong interfaces with the substrate and the Ni
3N layer [
38], which helps to alleviate the shear stress within the composite film and subsequently improves the bonding force between the film and the substrate [
39].
During the film deposition process, the arrangement of atoms and the formation of defects within the film are influenced by the bombardment and etching effects of energetic ions [
40,
41,
42,
43,
44], the energy imparted by these ions facilitates the diffusion of surface atoms, which aids in reducing the stress concentration areas in Ni/Ni
3N composite films. This preferred orientation at the interface between Ni and Ni
3N grains optimizes the film’s microstructure, and it makes the Ni layer more uniform and denser and better resistant to deformation and wear. According to
Figure 5a,c, Ni
3N in SLF and DLF was partially convex and had a higher roughness than ILF, but the crystalline structure of the Ni
3N films in (0 0 2) was more stable. They can better resist the interference and damage of the external environment, thereby enhancing its fracture toughness. As observed in the cross-section morphology of the Ni/Ni
3N composite films depicted in
Figure 4, the XRD results indicate that the grain size of Ni decreases with an increasing number of laminates, while the H/E value, which is a measure of the material’s wear resistance, and the plastic deformation resistance coefficient H
3/E
2 increases with more laminates. This enhancement in the composite film’s overall mechanical properties is attributed to the Hall–Petch effect [
45,
46,
47,
48], where the movement of dislocations is impeded due to the reduction in grain size.
3.3. Corrosion Resistance of Composite Film
Figure 8 shows the electrochemical polarization curve of the experimental sample in a 0.35% NaCl solution. The calculation results of the self-corrosion potential and corrosion current density [
49] are shown in
Table 4. It can be seen from the above Figures and Tables that the corrosion tendency of the composite films with the three laminated structures is significantly reduced, among which the corrosion rate of the Ni/Ni
3N/Ni composite film (ILF) sample is relatively smaller, and the self-corrosion potential is increased by about 8 times compared with the magnesium alloy. Zhang [
50] and Wu [
51] prepared Al and Cr films on the surface of magnesium alloy, respectively, and only increased the self-corrosion potential of magnesium alloy to −1.361 V and −1.250 V, respectively. Both Ni and Ni
3N are corrosion-resistant components, so the composite film has very good corrosion resistance. When nickel serves as the outer layer of the composite film, it readily forms a compact oxide film that acts as a passive barrier, it is shown by the following chemical formula (1):
This effectively sequesters the corrosive medium and thereby significantly enhances the film’s corrosion resistance.
Figure 9 shows the Nyquist [
52] curve of electrochemical AC impedance spectroscopy (EIS). The AZ31 magnesium alloy and the three composite films all exhibit high-frequency arc resistance. Nevertheless, the capacitive arc radius of the composite film is much larger than that of the magnesium alloy. The results indicate that the deposition of composite film markedly improves the magnesium alloy’s corrosion resistance. The curvature radius of the capacitor arc in the high-frequency region of the composite film is large, indicating that the charge transfer resistance is large, which is not conducive to the occurrence of a corrosion chemical reaction, and is the most important factor affecting the corrosion resistance of the composite film. The arc radius of the three laminated composite films has little difference, indicating that all of them have similar corrosion resistance.
The corrosion resistance of Ni/Ni
3N composite films is influenced by several key factors, including the phase structure, electrochemical properties of both Ni and Ni
3N, and grain size. It can be seen from
Figure 5b that the grain size distribution of the Ni layer is uniform and the quality is dense, which can isolate the external medium well. The self-corrosion potential of Ni is approximately −0.25V. When exposed to corrosive media, a chemically stable passivation film forms on the surface of Ni [
53,
54], which increases its corrosion potential and exhibits strong corrosion resistance. As a transition metal nitride, Ni
3N is also known for its excellent corrosion resistance. The strong electron interaction at the heterogeneous interface in the Ni/Ni
3N composite films, deposited in situ by magnetron sputtering, can limit the diffusion of corrosive molecules at the interface to a certain extent [
39,
55,
56]. Concurrently, it refines the grain size, thereby optimizing the electrochemical properties of the composite films [
57]. The intimate combination between the Ni layer and the Ni
3N layer, as depicted in
Figure 4, along with the multi-layered structure of the Ni/Ni
3N composite film, effectively prevents the penetration of corrosive media into the substrate [
58,
59], and this structure also plays a role in hindering the diffusion of oxygen (O) and chloride ions (Cl
—), thereby enhancing the corrosion resistance of the Ni/Ni
3N composite film.
The analysis of the Potentiodynamic polarization curve and Nyquist diagram reveals that the Ni/Ni3N composite film, deposited via magnetron sputtering, possesses superior corrosion resistance. This is primarily attributed to the formation of a stable passive film, the in situ growth resulting in a fine microstructure, tight inter-layer bonding, and a phase composition that includes both amorphous and nanocrystalline phases. Collectively, these factors slow the transmission of corrosion currents and enhance the corrosion resistance of the Ni/Ni3N composite films.
In summary, compared with ILF, SLF, and DLF, the corrosion current density of the three composite films is not much different in the same electrolyte, and each composite film greatly improves the corrosion resistance of the magnesium alloy surface, while the wear resistance of the three composite films is the best DLF, and the comprehensive performance of DLF is the best among the three films.