1. Introduction
Periodontitis is an oral disease characterized by a chronic immunoinflammatory reaction triggered by dysbiotic microorganisms present in dental biofilm [
1]. It affects around 35% of the adult population [
2], leading to irreversible loss of the tooth-supporting structures and, eventually, the teeth themselves [
3]. Gingival tissue serves as the primary site for the cellular changes implicated in the pathogenesis of periodontal diseases, and dysregulation of the local inflammatory response in the gums facilitates both the onset and progression of periodontitis [
4].
Evidence of alveolar bone loss [
1], resulting from an uncontrolled inflammatory reaction in the periodontal tissues [
3], is a critical criterion for diagnosing periodontitis. In addition to inflammation and alveolar bone loss, periodontitis is characterized by a state of oxidative stress at the cellular level [
5]. This imbalance disrupts the equilibrium between anti- and pro-inflammatory mediators, leading to excessive production of free radicals, which further impairs the immune response and induces cell death [
6,
7].
Scaling and root planning, performed with manual or ultrasonic instruments, is considered the gold standard treatment for periodontitis. It improves clinical parameters, reduces bacterial load, limits the progression of the disease, and mitigates systemic health risks [
8,
9]. Scaling and root planning has the capability to diminish the bacterial load and the inflammation caused by microbial activity. Notably, while this procedure seeks to establish a suitable environment for tissue reattachment, it can also, due to its invasive aspects, traumatize the inflamed periodontal tissues, leading to discomfort during probing, as well as inflammatory mediators generated in periodontal tissues, that can enter the bloodstream, resulting in systemic effects [
7].
Photobiomodulation (PBM) is a therapeutic technique that involves the application of precise doses of laser light energy, produced by red and near-infrared wavelengths within the electromagnetic spectrum, often termed the therapeutic window [
8]. This photonic energy is absorbed by cytochrome C oxidase, situated on the outer membrane of the mitochondria. Similarly, it reduces the levels of inflammatory mediators such as prostaglandin E2 (PGE2), tumor necrosis factor-alpha (TNFα), cyclooxygenase-2 (COX-2), the receptor activator of nuclear factor kappa-Β ligand/osteoprotegerin ratio (RANKL/OPG), interleukin-1 beta (IL-1β), and interferon-gamma (IF-γ). This leads to improved tissue oxygen diffusion, thereby facilitating the tissue repair process [
10,
11,
12].
Specifically, in periodontal tissues, PBM has been shown to reduce inflammation and pain [
13] and to promote tissue repair following surgical procedures [
14,
15,
16]. However, the efficacy of this therapy depends on several parameters, including the laser wavelength, the energy dose administered, and the condition of the irradiated tissue [
17].
The objective of the present study was to evaluate the effects of a PBM protocol on reactive oxygen species (ROS) and apoptosis in gingival tissue, as well as on markers of systemic inflammation and oxidative stress, in an animal model of experimental periodontitis.
4. Discussion
Our findings demonstrated that, in the experimental model used herein, photobiomodulation therapy attenuated ROS production and apoptosis in gingival tissue and reduced systemic inflammation and the pro-oxidant status. While several studies have utilized gingival tissue from experimental models of periodontitis to assess inflammatory and oxidative parameters [
4,
20], this is the first study to analyze the levels of superoxide anion, hydrogen peroxide, and percent apoptosis in gingival tissues affected by periodontitis, and assess the impact of an adjunctive PBM protocol. This contextualizes our work as an important research framework.
PBM is a noninvasive technique that has been investigated as an advantageous treatment option for several oral conditions, including periodontal disease [
14]. Our measurement of ROS levels in gingival tissue demonstrated that PBM was able to reduce these markers compared to the untreated group. Modulating inflammatory and oxidative processes in periodontal tissues through the antioxidant potential of PBM suggests a protective effect of laser therapy against cell and tissue damage in periodontitis. This finding aligns with previous in vitro studies that detected reductions in ROS [
22,
28,
29]. However, it is important to note that Rupel et al. (2018b) [
17] reported that a wavelength lower than that used in our study does not produce any effect in cells exposed to lipopolysaccharides (LPS) and can increase ROS levels in healthy cells. Additionally, Chen et al. (2011) [
30] and Wang et al. (2022) [
31] reported that high laser energy densities can lead to increased ROS release. Despite the positive effects of PBM observed in experimental and in vitro studies, clinical studies demonstrating its potential to reduce oxidative stress in periodontal tissues are still lacking.
Neutrophils and macrophages produce superoxide anions as part of the defense mechanism against pathogens, and elevated levels can disrupt tissue organization in periodontitis. Our results demonstrated that PBM regulates superoxide anions, which may suggest modulation of the immune response by reducing ROS levels, thereby minimizing tissue damage and controlling chronic inflammation. As a result, periodontal tissue integrity could be preserved, contributing to the maintenance of oral homeostasis.
We observed that in the PBM-treated group, apoptosis levels remained lower than in the group of animals with periodontitis treated with basic periodontal therapy alone. The modulation of ROS decreases apoptosis, thereby facilitating the repair of affected tissues [
32]. Faria et al. (2020) [
33] confirmed that PBM coordinates morphological, molecular, and biochemical changes within the cell to produce a reparative effect, by regulating cell death-related proteins, which can alter the permeability of the outer mitochondrial membrane, thus modulating both the intrinsic and extrinsic pathways of apoptosis. At a time when new perspectives are emerging regarding the role of mitochondrial dysfunction in periodontitis [
12,
34], PBM appears to play a crucial role as an adjunct to conventional periodontal therapies. Photobiomodulation’s action on mitochondria stimulates the synthesis of adenosine triphosphate (ATP) and can influence ROS regulation in cells and tissues undergoing oxidative stress [
12,
30,
35]. This suggests that PBM has the potential to address underlying cellular mechanisms contributing to periodontal disease and may enhance the effectiveness of conventional treatment strategies.
The laser, absorbed by mitochondria via cytochrome C oxidase, can enhance enzymatic activity by increasing oxygen consumption and stimulating adenosine triphosphate (ATP) synthesis through the photodissociation of inhibitory nitric oxide. Superoxide production in the mitochondria likely activates superoxide dismutase (SOD), converting it into hydrogen peroxide, which can cross mitochondrial membranes and activate beneficial signaling pathways. The modulation of ROS by PBM, involving anti-inflammatory mechanisms, may not be the only explanation. Other signaling pathways, such as those involving nitric oxide, cyclic adenosine monophosphate (AMP), and calcium, may also play a significant role in reducing inflammation, though further investigation is needed [
36]. Additionally, PBM may influence key molecular pathways such as nuclear factor kappa-B (NF-κB), which plays a crucial role in regulating inflammatory gene expression, and nuclear factor erythroid 2-related factor 2 (Nrf2), which is responsible for upregulating antioxidant defenses. By modulating these pathways, PBM could exert both anti-inflammatory and antioxidant effects, contributing to tissue protection and repair [
37,
38]. This expanded view aligns with the existing literature and provides a more comprehensive understanding of PBM’s mechanisms of action in the context of periodontal inflammation.
Our investigation revealed a reduction in levels of inflammatory activity and protein oxidation, indicating the systemic anti-inflammatory and antioxidant potential of PBM in periodontitis. Experimental studies examining the effect of PBM on MPO activity in periodontitis have reported findings like ours [
21,
27,
39]. Increased AOPP levels typically signify advanced oxidative stress and are linked to various systemic chronic inflammatory conditions [
40]. Additionally, previous research by Rupel and Ottaviani (2018a) [
41] demonstrated a reduction in AOPP levels in the gingival crevicular fluid (GCF) of patients undergoing periodontal treatment with adjunctive PBM. These findings underscore the potential systemic benefits of PBM as an adjunctive therapy for periodontitis.
We observed a modulating effect of PBM on pro-inflammatory and anti-inflammatory cytokines, characterized by a reduction in IL-6 and IL-12p70 and an increase in IL-10 levels. Additionally, we noted a significant difference in the IL-6/IL-10 ratio, indicating a potential systemic anti-inflammatory effect in our experimental model. However, cytokines such as IFN-γ and TNF-α, which are also involved in periodontitis development and are recognized in the literature for their pro-inflammatory profile [
42], did not show significant changes. This finding could be attributed to species-specific responses, as animal models may exhibit differences from humans due to the complex bacterial load associated with the disease.
It is important to acknowledge that the inflammatory response in periodontitis is multifaceted, involving numerous mediators. The lack of significant changes in IFN-γ and TNF-α does not necessarily reflect PBM’s ineffectiveness; rather, it may indicate that PBM’s mechanisms of action do not target these specific cytokines. One possible explanation is that T helper 1 (Th1) cells produce IFN-γ and TNF-α, which activate macrophages and stimulate IgG2a production, mediating a macrophage-dominant host defense response [
43].
This reduction in pro-inflammatory cytokine levels provides further evidence of the role of PBM in modulating inflammation. PBM can promote modulation in key cells of the inflammatory response, such as neutrophils, macrophages, and lymphocytes, by inhibiting pro-inflammatory signaling pathways, regulating oxidative stress and ROS production, and enhancing blood microcirculation and tissue oxygenation. Through these modifications, PBM can alter the inflammatory profile and response. Previous in vitro studies [
44,
45] have demonstrated the beneficial regulatory effect of PBM on cytokine secretion, shifting it from a pro-inflammatory to an anti-inflammatory profile. However, clinical studies have reported divergent results regarding the effect of PBM on cytokine levels in the context of periodontal treatment with different application protocols. While some studies have reported findings like ours [
46], others have described an absence of effect of PBM on cytokine regulation [
47,
48], underscoring the need for further research.
The results we obtained in the periodontitis group align with findings from previous studies [
12,
27,
34] regarding the mechanisms of oxidative stress involved in the progression of periodontal disease. Increased ROS levels, including hydrogen peroxide, and oxidation, contribute to mitochondrial dysfunction [
12], cell death [
49], and, clinically, periodontal attachment loss [
27]. Oxidative stress also entails systemic pro-inflammatory cytokines such as IL-6 and IL-12 [
50].
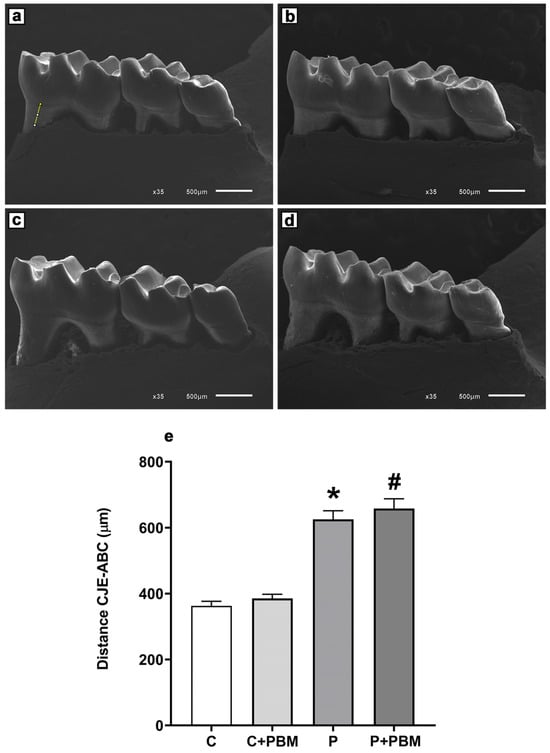
The PBM protocol utilized in our study achieved a steady state for some of the parameters of interest. However, the challenge of achieving an optimal anti-inflammatory dose and stimulating physiological homeostasis remains a weakness of PBM therapy [
51], given its biphasic dose–response relationship [
35] and its ability to alter host defense mechanisms dependent on dose, exposure time, and energy intensity [
17,
35]. A limitation of this study is that the methodology employed, with euthanasia conducted one day after the final laser application, did not allow for the evaluation of the potential long-term therapeutic effects of the laser. However, based on the beneficial effects observed in gingival cells, future investigations are needed to elucidate the effects of this PBM protocol on bone repair in the context of experimental periodontitis as well. To address this limitation, future studies should extend the observation period to evaluate the sustained impact of PBM on both tissue regeneration, bone, and the inflammatory response. Additionally, different experimental protocols, with varied application frequencies and dosages could help optimize PBM’s long-term benefits. Previous clinical studies have also indicated the need for prolonged evaluations, as bone repair outcomes have not been consistently observed despite extended laser use [
46,
47,
48]. Incorporating longer follow-up periods in both preclinical and clinical studies is crucial to better understand the therapeutic potential of PBM in managing periodontitis over time. Our findings provide further evidence of the promising role of PBM as an adjunct to conventional periodontal therapy. PBM has the potential to mitigate both local and systemic inflammatory and oxidative processes involved in the pathogenesis of periodontitis.