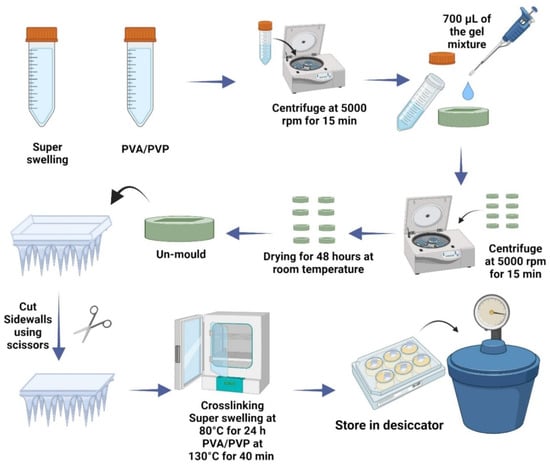
1. Introduction
The field of transdermal drug delivery and biosensing has witnessed significant progresse with the development of hydrogel microneedles (HMNs), a minimally invasive technology that enables controlled drug release, interstitial fluid (ISF) extraction, and real-time biomarker detection. Unlike traditional hypodermic needles or passive transdermal patches, HMNs leverage hydrogel-forming polymers to penetrate the skin, absorb ISF, and facilitate sustained drug diffusion or biosensing interactions [
1,
2,
3]. These systems provide several advantages over conventional delivery and diagnostic platforms, including enhanced patient compliance, reduced systemic toxicity, and improved drug bioavailability, making them highly suitable for chronic disease management, vaccination, wound healing, and cancer therapy [
1,
4,
5,
6,
7,
8].
The concept of microneedles dates back to the early 20th century, with Dr. Ernst Kromayer pioneering microneedle-like techniques in dermatology for scar treatment and pigmentation disorders. The first mention of microneedle use appeared in 1921, while interest in transdermal drug delivery through microneedles began to grow in the 1960s. However, it was not until the late 1990s that microfabrication advancements enabled the development of silicon microneedles for enhanced drug delivery [
9]. Since then, microneedle technology has rapidly evolved, incorporating diverse materials, fabrication techniques, and biomedical applications, paving the way for modern HMNs.
The primary mechanism of HMNs involves polymer-based microneedle arrays that swell upon contact with skin moisture, forming a conduit for therapeutic agents or biomarker analysis [
5,
10,
11,
12]. This technology allows for tunable mechanical properties, precise drug-loading capabilities, and multi-responsive release kinetics. Moreover, the integration of smart and stimuli-responsive hydrogels has enabled on-demand drug administration, responding to physiological cues such as pH, glucose fluctuations, or enzymatic activity [
4,
13,
14]. In diagnostics, HMNs have emerged as a powerful alternative to invasive blood sampling techniques, offering continuous glucose monitoring, electrochemical biosensing, and smartphone-integrated biomarker detection [
15,
16,
17].
The rapid expansion of microneedle-based technologies has led to a surge in academic interest, with research increasingly focusing on their potential in transdermal drug administration, vaccines, and biomolecule delivery for skin-related conditions [
18]. While their benefits are well-documented, challenges remain regarding scalability, mechanical stability, and drug-loading capacities. Addressing these limitations early on is crucial for optimizing the clinical translation of microneedle platforms.
HMNs are fabricated using a variety of techniques, including micromolding, photopolymerization, 3D printing, and enzyme-mediated crosslinking, each tailored to optimize mechanical integrity, drug retention, and biosensing efficiency [
19,
20,
21,
22]. Recent advancements have further expanded the scope of microneedle applications, incorporating various types such as solid, coated, hollow, dissolvable, hydrogel, swellable, and porous microneedles, each with unique properties depending on materials and fabrication methods [
23]. The versatility of microneedles extends beyond drug delivery to include diagnostics, tissue engineering, cancer research, and wound care, making them a promising tool in modern biomedical applications.
Advanced material engineering has also introduced nanoparticle-enhanced HMNs, integrating gold nanoparticles, graphene oxide, and metal–organic frameworks (MOFs) to improve drug solubility, bioavailability, and precision targeting [
15,
24,
25]. Additionally, wearable and wireless MN patches are being developed to facilitate real-time health monitoring, with potential applications in chronic disease management and point-of-care diagnostics [
17,
26]. Emerging precision medicine strategies, such as the acupoint–target-organ–ganglion approach, have further refined drug targeting, improving therapeutic efficacy through specific drug concentration at intended sites [
27]. Innovations in biocompatible materials and mechanical properties are expected to enhance microneedle performance while ensuring patient safety and comfort.
Despite their advantages, the widespread clinical translation of HMNs remains hindered by challenges in drug release precision, mechanical stability, scalability, biosensing accuracy, and regulatory approval [
28,
29,
30,
31,
32,
33]. Issues such as burst release, unintended drug leakage, and inconsistent swelling behavior affect performance, while manufacturing complexities and high production costs limit commercialization. Additionally, regulatory frameworks for combination drug–device MN systems remain undefined, slowing clinical adoption and necessitating large-scale multi-center trials to validate efficacy and safety.
This review presents a comprehensive analysis of the key materials, fabrication strategies, biosensing capabilities, and drug delivery mechanisms of HMNs. It further explores their limitations, regulatory considerations, and emerging trends, emphasizing the potential of next-generation hybrid, AI-driven, and personalized MN platforms to transform transdermal therapeutics and diagnostics.
2. Key Materials for HMNs
HMNs’ success in transdermal drug delivery and diagnostics depends largely on the composition of the hydrophilic polymers, crosslinkers, nanoparticles, and functional bio-additives that form their structure. These materials determine the mechanical properties, drug-loading efficiency, biodegradability, and responsiveness to stimuli.
2.1. Crosslinked and Hydrophilic Polymers: The Backbone of HMNs
HMNs rely on hydrophilic polymers for their mechanical strength, swelling properties, and controlled drug release. These polymers ensure that the microneedles can penetrate the skin, absorb interstitial fluid, and deliver therapeutic agents effectively.
2.1.1. Synthetic Polymers for Structural Integrity and Drug Delivery
Synthetic polymers provide stability, tunability, and high drug-loading capacity in HMNs. Poly(ethylene glycol) (PEG), available in molecular weights of 10,000 Da, enhances hydrophilicity and flexibility, ensuring optimal drug diffusion through the microneedle matrix [
1,
34]. Similarly, poly(methyl vinyl ether-co-maleic acid) (PMVE/MA) with sodium bicarbonate significantly improves swelling behavior and drug retention, making it ideal for sustained-release formulations [
1,
10].
Poly(acrylic acid-co-maleic Acid) (PAMA) and polyvinyl alcohol (PVA) (1:4 ratio, crosslinked at 90 °C for 30 min) create a stable matrix that swell upon contact with skin moisture, allowing for rapid drug release [
35]. Additionally, methacrylated hyaluronic acid (MeHA) is widely used for biofilm penetration, aptamer-based biosensing, and targeted drug delivery due to its excellent biocompatibility and modification flexibility [
36].
2.1.2. Natural and Hybrid Polymers for Biocompatibility
Natural polymers enhance the biocompatibility and biodegradability of HMNs. Silk fibroin methacrylate provides a strong structural network while enabling the delivery of bioactive molecules such as alpha-MSH, a treatment for vitiligo [
37]. Additionally, methacryloyl chitosan (CSMA) hydrogels, on the other hand, are widely used for psoriasis treatment, allowing for the controlled release of methotrexate and nicotinamide [
38].
2.2. Functionalized Nanoparticles and Crosslinking Agents for Enhanced Performance
HMNs are often reinforced with crosslinkers and nanoparticles to improve mechanical strength, drug-loading efficiency, and response to stimuli.
2.2.1. Crosslinking Agents for Structural Reinforcement
Chemical crosslinking enhances HMNs’ mechanical properties, ensuring they can penetrate the skin without breaking. Na
2CO
3 (3%
w/
w) plays a crucial role in regulating pH and modifying crosslinking density to optimize swelling and stability [
1]. Dopamine-functionalized hydrogels contribute to biosensing capabilities by facilitating redox-based diagnostics [
26].
For high-dose drug delivery, Gantrez S-97, PEG (10,000 Da), and Na
2CO
3 are combined to produce super-swelling HMNs, which provide the rapid absorption of interstitial fluid and controlled release of therapeutic agents [
39]. Phenylboronic acid-based hydrogels introduce an additional level of control by enabling glucose-responsive insulin delivery through reversible phenylborate ester crosslinking, making them highly effective for diabetes treatment [
13,
40]. An aqueous blend of poly(vinyl alcohol) (PVA), polyvinylpyrrolidone (PVP), crosslinked with citric acid, was able to deliver methotrexate, albendazole, and sildenafil citrate to treat different disease conditions [
41,
42,
43].
2.2.2. Functionalized Nanoparticles for Targeted Therapy
Nanoparticles improve HMNs’ ability to carry and release drugs in a controlled manner. Similarly, tetrakis(1-methyl-4-pyridinio)porphyrin (TMPyP)-loaded PLGA nanoparticles inside enzyme-mediated hyaluronic acid-tyramine (HAT) hydrogels enable photodynamic therapy in melanoma treatment by targeting cancer cells with light-activated drug release [
4]. For antibiotic-resistant infections, mono-(6-diethylenetriamine-6-deoxy)-beta-cyclodextrin (mbeta-CD) is incorporated into HMNs to deliver celastrol, which has potent antimicrobial properties [
12]. Dopamine-conjugated hyaluronic acid hydrogels integrated with PEDOT:PSS and Ag-Pt nanoparticles further enable real-time glucose monitoring and pH biosensing, improving diabetes management and metabolic disorder tracking [
15,
30].
2.3. Smart and Responsive Polymers for Controlled Drug Release and Biosensing
Smart polymers in HMNs introduce responsiveness to physiological conditions, allowing for precise drug release based on pH, temperature, or external stimuli.
2.3.1. Temperature- and pH-Responsive Polymers
Poly(N-Isopropylacrylamide) (pNIPAM) undergoes phase transitions at body temperature, enabling controlled insulin release for diabetic patients without the need for continuous injections [
44,
45]. For inflammation treatment, taurine-loaded Prussian blue nanoparticles in methacrylate-based hyaluronic acid HMNs are designed to release anti-inflammatory agents upon exposure to acidic and photothermal stimuli, making them useful for chronic wound healing [
46].
2.3.2. Electrically and Light-Responsive Hydrogels
Electrically conductive and light-sensitive HMNs provide additional control over drug delivery. HMNs containing black phosphorus (BP) microspheres and pNIPAM introduce near-infrared (NIR)-triggered drug release for precise insulin administration in diabetic patients [
44]. Moreover, HMNs composed of polydopamine@polypyrrole enable light-responsive photothermal effects by absorbing near-infrared light and converting it into heat for killing bacteria [
47].
2.4. Hybrid Hydrogels for Sustained Drug Release
A hybrid form of HMN provides a controlled and prolonged drug release profile while minimizing toxicity and adverse effects.
2.4.1. Crosslinked Hybrid Polymers
Poly(lactide-co-glycolide) (PLGA) tips combined with poly(vinyl alcohol) (PVA) and polyvinylpyrrolidone) (PVP) hydrogel bases create hybrid microneedles for sustained amphotericin B release, improving antifungal treatments [
48]. Poly(methylvinylether-co-maleic acid) crosslinked with pectin enhances bioadhesion and controlled drug diffusion, making it useful for transdermal drug administration [
11].
2.4.2. Drug Reservoirs and Inclusion Complexes
To improve drug solubility, beta-cyclodextrin drug reservoirs are integrated into HMNs for delivering telmisartan for treating hypertension and curcumin for anticancer therapy [
49,
50]. HMNs embedded in collagen type-I cryogels are optimized for ocular drug delivery, providing antibacterial treatment for eye infections [
51]. Additionally, HMNs also used several other reservoir systems such as PEG reservoir, lyophilized reservoir, and compressed tablet reservoir [
2,
49,
52].
Table 1 presents key materials and additives used in HMNs, highlighting their notable properties. Synthetic polymers such as PVA, PVP, PEG, and Gantrez S-97 dominate, offering mechanical strength, swelling ability, and biocompatibility. Natural polymers like chitosan, alginate, and hyaluronic acid (HA) emphasize biodegradability and bioadhesion. Crosslinked materials (e.g., MeHA, GelMA, DexMA) provide enhanced gel stability and tunable mechanical properties. Stimuli-responsive polymers, such as pNIPAAm and Carbopol, enable temperature- and pH-sensitive drug release. Nanomaterials (graphene oxide, silver nanoparticles) contribute conductivity and antimicrobial properties. Overall, the table highlights a balance between biocompatibility, mechanical strength, and smart drug release properties, suggesting a trend toward hybrid, responsive, and multifunctional HMN formulations.
11. Conclusions
HMNs represent a paradigm shift in transdermal drug delivery and biosensing, combining biocompatibility, precision drug release, and non-invasive biomarker monitoring. Despite their immense potential, challenges in stability, manufacturing scalability, and regulatory approval hinder their clinical integration. Advances in hybrid biomaterials, smart-responsive drug release, and AI-powered biosensing platforms offer promising solutions to these limitations. Future research must focus on multi-functional MNs, personalized medicine, and large-scale clinical validation to accelerate commercialization. As HMNs technology evolves, its integration into real-time health monitoring, chronic disease management, and controlled immunotherapy will redefine patient care. Bridging material innovation with regulatory advancements is the key to unlocking the full potential of HMNs in next-generation biomedical applications.