1. Introduction
Table 1.
Characteristics of MYH6 variants included in the study. Allele frequencies were obtained from the Genome Aggregation Database Genomes dataset (gnomAD) v4.1.0. Combined annotation-dependent depletion (CADD) scores are based on genome build GRCh38 (CADD v1.7).
Table 1.
Characteristics of MYH6 variants included in the study. Allele frequencies were obtained from the Genome Aggregation Database Genomes dataset (gnomAD) v4.1.0. Combined annotation-dependent depletion (CADD) scores are based on genome build GRCh38 (CADD v1.7).
| Study ID | MYH6 Variant | dbSNP ID | Allele Frequency | CADD | Previously Reported |
|---|---|---|---|---|---|
| R0735 | Q277H | rs140660481 | 2.6 × 10−4 | 25.0 | Y [6,14,15] |
| 21_124 | A336G | rs138572790 | 6.8 × 10−5 | 23.7 | N |
| 07_155 | D383N | N/A | Not reported | 25.7 | Y [6] |
| 10_121 | S385L | rs778319108 | 5.9 × 10−5 | 24.1 | Y [6] |
| 10_121 | M436V | N/A | 6.2 × 10−7 | 24.9 | Y [6] |
| 07_067 | R443P | N/A | Not reported | 28.7 | Y [6,16] |
| R_0622 | F469V | N/A | Not reported | 26.0 | N |
| 18_001 | K867R | rs143284278 | 8.7 × 10−6 | 24.5 | N |
| 07_074 | K849- | N/A | N/A | N/A | Y [6] |
| R0121 | A936V | rs199838024 | 2.7 × 10−4 | 24.6 | Y [6,17] |
| 10_249, 09_299 | A964S | rs144907522 | 2.2 × 10−4 | 24.7 | Y [6] |
| 07_082 | R1151Q | rs745406670 | 2.7 × 10−5 | 28.0 | Y [6] |
| 09_103 | A1298V | rs368588052 | 1.4 × 10−4 | 25.9 | Y [6] |
| 09_152, 12_093 | T1379M | rs145611185 | 8.6 × 10−4 | 31 | Y [6,18,19,20] |
| 11_003 | A1443D | rs727503234 | 2.0 × 10−4 | 26.2 | Y [6,14,19,21] |
| 12_234 | E1503V | N/A | Not reported | 35.0 | Y [6] |
| 09_204 | E1584K | rs1280321639 | 2.5 × 10−6 | 28.7 | Y [6] |
| 07_026 | E1754X | rs372270600 | 1.9 × 10−6 | 45.0 | Y [6] |
| R0300 | K1840R | rs373629059 | 1.1 × 10−4 | 28.2 | Y [6,22] |
2. Materials and Methods
2.1. Study Population
2.2. Echocardiogram Acquisition
All the echocardiograms were assessed retrospectively. Studies were obtained from awake patients shortly after birth, prior to any surgical intervention. The echocardiogram clip duration and frame rates varied based on age and quality of study.
2.3. Myocardial Strain
All the measurements were obtained from the apical four-chamber view using a semi-automated tracking method. Users first delineated the endocardial border at end-diastole and the TomTec tracking algorithm was allowed to calculate myocardial deformation. The accuracy of the tracking was reviewed by a pediatric cardiology faculty member and if needed, the borders were adjusted manually. Global longitudinal strain (GLS) and time-plots for strain and strain rate (SR) were reported by TomTec.
2.4. Statistical Analysis
Poor outcomes were defined as the need for mechanical circulatory support, cardiac transplant, or death. Categorical variables are reported as N(%) and groups compared using an exact Fisher test. Continuous variables are reported as median (IQR), unless otherwise indicated, and compared using exact two-tailed nonparametric Mann-Whitney tests. Pearson correlation coefficients were calculated for the linear association of the strain parameters with heart rate, and two-tailed significance was obtained. A general linear model with a normal error link was used to look further at associations between strain and heart rate and the R2 was adjusted for these two variables. The 15-year event-free survival between the groups was compared using Kaplan-Meier curves, and the significance was evaluated using a logrank test. For all the analyses, p < 0.05 was considered significant but given the small sample size, we considered p < 0.1 to be a trend towards significance. No adjustment for multiple comparisons was made when examining our primary outcome of interest (ASct).
3. Results
3.1. Characteristics of the Study Cohort
To the extent possible in a retrospective study, controls were selected that were born in similar eras as variant carriers; the median time between the date of birth of cases and matched controls was 1.58 years (IQR 0.83, 2.42). The median age at the time of the pre-surgical echocardiogram was 0.0 days for both variant carriers (IQR 0.0, 2.0; maximum 7.0) and controls (IQR 0.0, 1.0; maximum 13.0). Most patients were in sinus rhythm at the time of the echocardiograms; a junctional rhythm was suspected in one patient but could not be confirmed. Most had heart rates within expected ranges for the newborn period (120–190 bpm); one was slightly bradycardic (102 bpm).
3.2. Event-Free Survival
3.3. Right Atrial and Ventricular Strain Analyses
4. Discussion
4.1. Right Atrial Contractile Function
4.2. Right Atrial Conduit and Reservoir Function
4.3. Impact of Right Ventricular Function
4.4. Limitations
Our study did have some notable limitations. Assessing echocardiograms obtained prior to any surgical intervention is our best opportunity to examine native RA mechanics, as once staged palliation begins many factors impact both atria and overall cardiac function, potentially confounding the influence of MYH6 variants. However, the retrospective nature of the study may have resulted in suboptimal echocardiogram views and variation in image quality over the years, including a lack of tricuspid color Doppler on many of the studies. While we were able to minimize some of these differences between the groups by control-matching based on approximate year of birth, this also limited our ability to match based on atrial restriction or account for the degree of tricuspid regurgitation.
5. Conclusions
Our findings demonstrate atrial dysfunction associated with MYH6 variants in HLHS. HLHS patients with MYH6 variants have decreased atrial contractile strain, as well as an increase in reservoir and conduit strain with increasing HR. RV strain indices were not decreased in MYH6 variant carriers, consistent with a primary atrial phenotype.
The confirmation of these findings in a larger cohort, along with longitudinal studies of atrial function in HLHS, are warranted to identify if RA dysfunction in MYH6 variant carriers is evident throughout the lifetime of these patients and, if so, its relationship to decreased transplant-free survival. Such studies will further elucidate if atrial dysfunction could be an informative prognostic consideration in HLHS patients with MYH6 variants and thus justify early genetic screening.
Author Contributions
M.Q.A., conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review & editing; S.C., conceptualization, investigation, methodology, visualization, writing—original draft, writing—review & editing; P.M.S., formal analysis, methodology, writing—review & editing; J.M.J., conceptualization, methodology, resources, project administration, supervision; P.L., data curation, validation; P.C.F., conceptualization, methodology, resources, project administration, software; A.T.-M., conceptualization, funding acquisition, project administration, resources, supervision, writing—review & editing; M.E.M., conceptualization, funding acquisition, project administration, resources, supervision, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the MCW Advancing a Healthier Wisconsin Endowment, the MCW CTSI via NIH CTSA awards (UL1TR001436, 1TL1TR001437), the MCW Department of Surgery, and the Herma Heart Institute. MQA is a member of the MCW Medical Scientist Training Program, which is partially supported by a NIGMS training grant (T32-GM080202).
Institutional Review Board Statement
All the studies were performed in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments, as well as local and institutional ethical regulations. All the protocols were approved by the MCW Institutional Review Board (PRO ID #00047811, approval date 10 October 2023).
Informed Consent Statement
Written informed consent, and assent where applicable, was obtained from all participants for the collection and use of information (including deidentified genetic information, medical records and images, including echocardiograms, cardiac MRI, ECG, and Holter monitoring) for research purposes, including publication.
Data Availability Statement
Restrictions apply to the availability of these data. The data are restricted as they contain protected patient information.
Acknowledgments
We thank the families, physicians, and clinical care team of the Herma Heart Institute, including Tracy Geoffrion, MD, MPH, Megan Schloessing, and the HHI echocardiography research lab, Mary Krolikowski, Anne Laulederkind, Regina Cole, Mary Goetsch, MS, Huan-Ling Liang, MD, and Lisa Armitage for their support.
Conflicts of Interest
No author relationships with industry are directly related to the material in this publication. Other industry relationships of authors include: MEM, ATM are founders, major stockholders, board members, and receive research support from TAI Diagnostics. MEM, ATM are founders and stockholders of MD Interactive. MEM, ATM have relationships with GenDx (intellectual property) and Natera (research support, intellectual property).
References
- Ohye, R.G.; Schranz, D.; D’Udekem, Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016, 134, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, B.; Deshpande, S.; McCracken, C.; Kogon, B.; Vincent, R.; Mahle, W.; Kanter, K. Results of heart transplantation following failed staged palliation of hypoplastic left heart syndrome and related single ventricle anomalies. Eur. J. Cardiothorac. Surg. 2015, 48, 792–798; discussion 798–799. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, B.; Mahle, W.T.; Manlhiot, C.; Deshpande, S.; Kogon, B.; McCrindle, B.W.; Kanter, K. Outcomes of heart transplantation in children with hypoplastic left heart syndrome previously palliated with the Norwood procedure. J. Thorac. Cardiovasc. Surg. 2016, 151, 167–174, 175, e161–162. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Bird, T.M.; Jaquiss, R.D.; Morrow, W.R.; Robbins, J.M. Comparison of in-hospital and longer-term outcomes of hybrid and Norwood stage 1 palliation of hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2015, 150, 474–480.e472. [Google Scholar] [CrossRef]
- Tomita-Mitchell, A.; Stamm, K.D.; Mahnke, D.K.; Kim, M.S.; Hidestrand, P.M.; Liang, H.L.; Goetsch, M.A.; Hidestrand, M.; Simpson, P.; Pelech, A.N.; et al. Impact of MYH6 variants in hypoplastic left heart syndrome. Physiol. Genomics 2016, 48, 912–921. [Google Scholar] [CrossRef]
- Anfinson, M.; Fitts, R.H.; Lough, J.W.; James, J.M.; Simpson, P.M.; Handler, S.S.; Mitchell, M.E.; Tomita-Mitchell, A. Significance of α-Myosin Heavy Chain (MYH6) Variants in Hypoplastic Left Heart Syndrome and Related Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 144. [Google Scholar] [CrossRef]
- Reiser, P.J.; Portman, M.A.; Ning, X.H.; Moravec, C.S. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1814–H1820. [Google Scholar] [CrossRef]
- Hove, J.R.; Koster, R.W.; Forouhar, A.S.; Acevedo-Bolton, G.; Fraser, S.E.; Gharib, M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421, 172–177. [Google Scholar] [CrossRef]
- Dietrich, A.C.; Lombardo, V.A.; Veerkamp, J.; Priller, F.; Abdelilah-Seyfried, S. Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev. Cell 2014, 30, 367–377. [Google Scholar] [CrossRef]
- Nadorlik, H.; Fleishman, C.; Brown, D.W.; Miller-Tate, H.; Lenahan, P.; Nicholson, L.; Wheller, J.; Cua, C.L. Survey of How Pediatric Cardiologists Noninvasively Evaluate Patients with Hypoplastic Left Heart Syndrome. Congenit. Heart Dis. 2015, 10, E73–E82. [Google Scholar] [CrossRef] [PubMed]
- Namana, V.; Gupta, S.S.; Sabharwal, N.; Hollander, G. Clinical significance of atrial kick. QJM 2018, 111, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Gorcsan, J., 3rd; Tanaka, H. Echocardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011, 58, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Hu, J.J.; Sundsbak, R.S.; Evans, J.M.; Bamlet, W.R.; Qureshi, M.Y.; O’Leary, P.W.; Olson, T.M. Genetic Association Between Hypoplastic Left Heart Syndrome and Cardiomyopathies. Circ. Genom. Precis. Med. 2021, 14, e003126. [Google Scholar] [CrossRef]
- Pulignani, S.; Vecoli, C.; Borghini, A.; Foffa, I.; Ait-Ali, L.; Andreassi, M.G. Targeted Next-Generation Sequencing in Patients with Non-syndromic Congenital Heart Disease. Pediatr. Cardiol. 2018, 39, 682–689. [Google Scholar] [CrossRef]
- Kim, M.S.; Fleres, B.; Lovett, J.; Anfinson, M.; Samudrala, S.S.; Kelly, L.J.; Teigen, L.E.; Cavanaugh, M.; Marquez, M.; Geurts, A.M.; et al. Contractility of induced pluripotent stem cell-cardiomyocytes with an MYH6 head domain variant associated with hypoplastic left heart syndrome. Front Cell Dev Biol. 2020, 8, 440. [Google Scholar] [CrossRef]
- Priest, J.R.; Osoegawa, K.; Mohammed, N.; Nanda, V.; Kundu, R.; Schultz, K.; Lammer, E.J.; Girirajan, S.; Scheetz, T.; Waggott, D.; et al. De Novo and Rare Variants at Multiple Loci Support the Oligogenic Origins of Atrioventricular Septal Heart Defects. PLoS Genet. 2016, 12, e1005963. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Zimmermann, M.T.; Evans, J.M.; Eckloff, B.W.; Wieben, E.D.; Qureshi, M.Y.; O’Leary, P.W.; Olson, T.M. Recessive MYH6 Mutations in Hypoplastic Left Heart with Reduced Ejection Fraction. Circ. Cardiovasc. Genet. 2015, 8, 564–571. [Google Scholar] [CrossRef]
- Granados-Riveron, J.T.; Ghosh, T.K.; Pope, M.; Bu’Lock, F.; Thornborough, C.; Eason, J.; Kirk, E.P.; Fatkin, D.; Feneley, M.P.; Harvey, R.P.; et al. Alpha-cardiac myosin heavy chain (MYH6) mutations affecting myofibril formation are associated with congenital heart defects. Hum. Mol. Genet. 2010, 19, 4007–4016. [Google Scholar] [CrossRef]
- van Wijngaarden, A.L.; Hiemstra, Y.L.; Koopmann, T.T.; Ruivenkamp, C.A.L.; Aten, E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Barge-Schaapveld, D.; Ajmone Marsan, N. Identification of known and unknown genes associated with mitral valve prolapse using an exome slice methodology. J. Med. Genet. 2020, 57, 843–850. [Google Scholar] [CrossRef]
- Granados-Riveron, J.T.; Pope, M.; Bu’lock, F.A.; Thornborough, C.; Eason, J.; Setchfield, K.; Ketley, A.; Kirk, E.P.; Fatkin, D.; Feneley, M.P.; et al. Combined mutation screening of NKX2-5, GATA4, and TBX5 in congenital heart disease: Multiple heterozygosity and novel mutations. Congenit. Heart Dis. 2012, 7, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Bozzao, C.; Pennacchini, E.; Pagannone, E.; Musumeci, B.M.; Piane, M.; Germani, A.; Savio, C.; Francia, P.; Volpe, M.; et al. A Next-Generation Sequencing Approach to Identify Gene Mutations in Early- and Late-Onset Hypertrophic Cardiomyopathy Patients of an Italian Cohort. Int. J. Mol. Sci. 2016, 17, 1239. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alfoldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Anfinson, M.; Kim, M.S.; Lough, J.; Geddes, G.; James, J.; Geurts, A.; Mitchell, M.; Tomita-Mitchell, A. A Novel MYH6E1503V Variant in a Family with a History of Heart Disease, including Hypoplastic Left Heart Syndrome. FASEB J. 2019, 33, 831.3. [Google Scholar] [CrossRef]
- Anfinson, M.; Thareja, S.; Cavanaugh, M.; Brown, R.; Kim, M.-S.; Liang, H.-L.; Mitchell, M.; Fitts, R.; Tomita-Mitchell, A. The novel MYH6-E1584K tail domain variant associated with hypoplastic left heart syndrome leads to hypercontractility in vitro. Physiology 2023, 38, 5733555. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Khoo, N.S.; Smallhorn, J.F.; Kaneko, S.; Kutty, S.; Altamirano, L.; Tham, E.B. The assessment of atrial function in single ventricle hearts from birth to Fontan: A speckle-tracking study by using strain and strain rate. J. Am. Soc. Echocardiogr. 2013, 26, 756–764. [Google Scholar] [CrossRef]
- Kutty, S.; Padiyath, A.; Li, L.; Peng, Q.; Rangamani, S.; Schuster, A.; Danford, D.A. Functional maturation of left and right atrial systolic and diastolic performance in infants, children, and adolescents. J. Am. Soc. Echocardiogr. 2013, 26, 398–409.e392. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Teramoto, R.; Nomura, A.; Asano, Y.; Beerens, M.; Kurata, Y.; Kobayashi, I.; Fujino, N.; Furusho, H.; Sakata, K.; et al. Impact of functional studies on exome sequence variant interpretation in early-onset cardiac conduction system diseases. Cardiovasc. Res. 2020, 116, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Klos, M.; Mundada, L.; Banerjee, I.; Morgenstern, S.; Myers, S.; Leone, M.; Kleid, M.; Herron, T.; Devaney, E. Altered myocyte contractility and calcium homeostasis in alpha-myosin heavy chain point mutations linked to familial dilated cardiomyopathy. Arch. Biochem. Biophys. 2017, 615, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, S.L.; Maniar, H.S.; Bloch, J.B.; Steendijk, P.; Moon, M.R. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 2005, 112, I212–I218. [Google Scholar] [CrossRef]
- Prioli, A.; Marino, P.; Lanzoni, L.; Zardini, P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am. J. Cardiol. 1998, 82, 756–761. [Google Scholar] [CrossRef]
- Wang, A.P.; Polsen, C.; Penk, J.; Husain, N.; Hauck, A.; Jone, P.N. Common atrial reservoir strain during the interstage period is a predictor of poor outcomes prior to Fontan completion in hypoplastic left heart syndrome. Echocardiography 2024, 41, e15910. [Google Scholar] [CrossRef]
- Boettler, P.; Hartmann, M.; Watzl, K.; Maroula, E.; Schulte-Moenting, J.; Knirsch, W.; Dittrich, S.; Kececioglu, D. Heart rate effects on strain and strain rate in healthy children. J. Am. Soc. Echocardiogr. 2005, 18, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Hakim, T.S.; Michel, R.P.; Chang, H.K. Effect of lung inflation on pulmonary vascular resistance by arterial and venous occlusion. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 53, 1110–1115. [Google Scholar] [CrossRef]
- Brower, R.; Wise, R.A.; Hassapoyannes, C.; Bromberger-Barnea, B.; Permutt, S. Effect of lung inflation on lung blood volume and pulmonary venous flow. J. Appl. Physiol. 1985, 58, 954–963. [Google Scholar] [CrossRef]
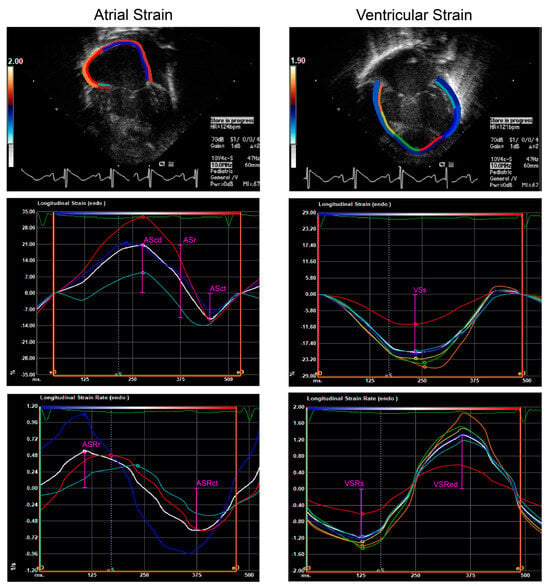
Representative tracings and time-rate plots of atrial and ventricular myocardium in postnatal HLHS. AScd, conduit atrial strain; ASr, reservoir atrial strain; ASct, active/contractile atrial strain; ASRr, reservoir atrial strain rate; ASRct, active/contractile atrial strain rate; VSs, systolic ventricular strain; VSRs, systolic ventricular strain rate; and VSRed, early diastolic ventricular strain rate.
Figure 1.
Representative tracings and time-rate plots of atrial and ventricular myocardium in postnatal HLHS. AScd, conduit atrial strain; ASr, reservoir atrial strain; ASct, active/contractile atrial strain; ASRr, reservoir atrial strain rate; ASRct, active/contractile atrial strain rate; VSs, systolic ventricular strain; VSRs, systolic ventricular strain rate; and VSRed, early diastolic ventricular strain rate.
Kaplan-Meier curves comparing 15-year event-free survival in MYH6 variant carriers vs. controls. Shaded areas represent 95% confidence intervals. ‘+’ signs indicate censored patients.
Figure 2.
Kaplan-Meier curves comparing 15-year event-free survival in MYH6 variant carriers vs. controls. Shaded areas represent 95% confidence intervals. ‘+’ signs indicate censored patients.
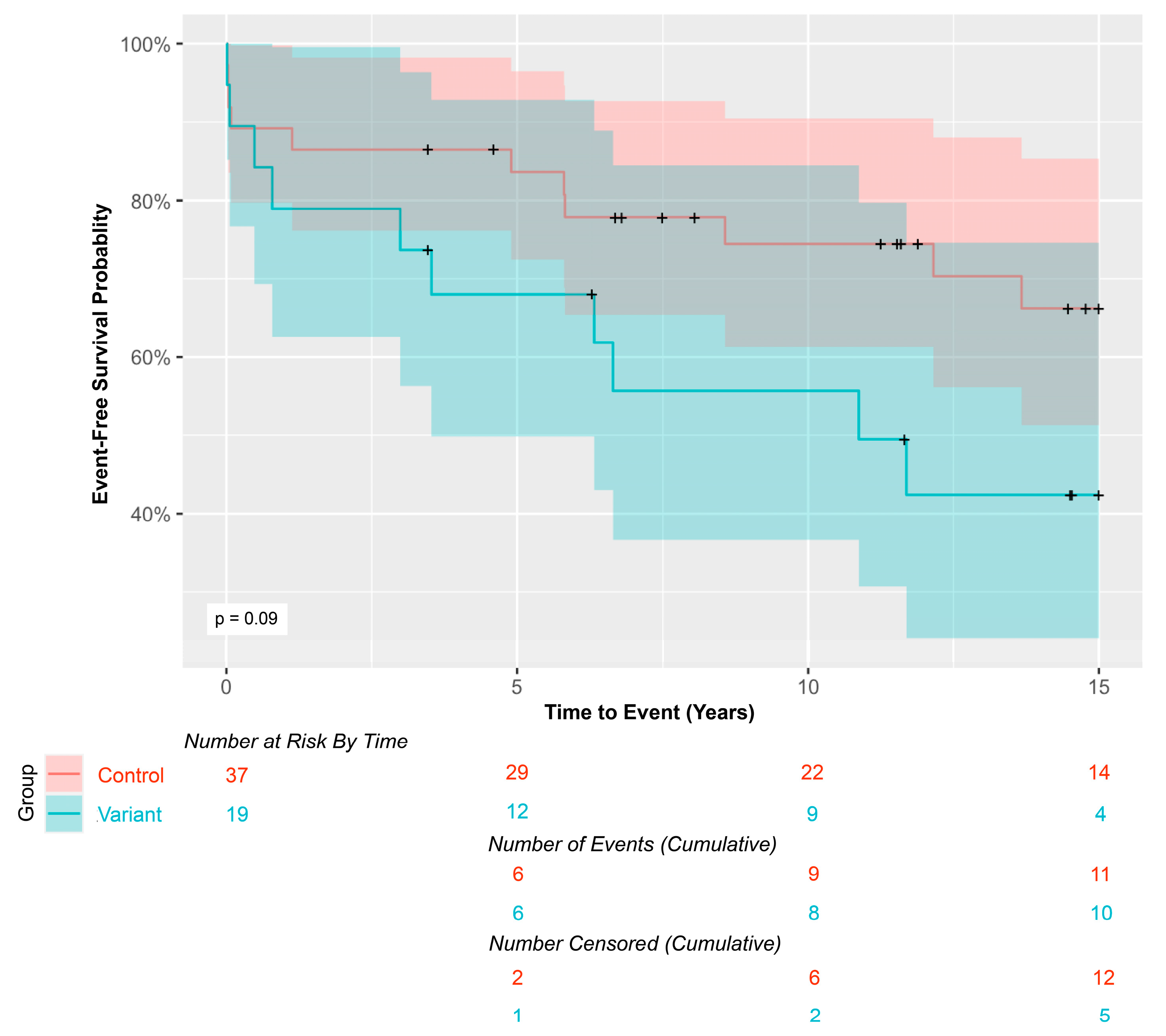
Table 2.
Patient characteristics.
Table 2.
Patient characteristics.
| MYH6 Variant n = 19 (%) | Control n = 37 (%) | |
|---|---|---|
| Sex | ||
| Male | 9 (47.4) | 20 (54.1) |
| Female | 10 (52.6) | 17 (45.9) |
| Anatomy | ||
| AA/MA | 10 (52.6) | 19 (51.4) |
| AA/MS | 2 (10.5) | 4 (10.8) |
| AS/MS | 7 (36.8) | 14 (37.8) |
| RAS or IAS | 7 (36.8) | 12 (32.4) |
| Respiratory Status | ||
| Mechanical ventilation | 5 (26.3) | 7 (18.9) |
| Room air or nasal cannula | 12 (63.2) | 29 (78.3) |
| Unknown | 2 (10.5) | 1 (2.7) |
| Stage I shunt type | ||
| BTTS | 10 (52.6) | 19 (51.4) |
| RVPAC (Sano) | 9 (47.4) | 18 (48.6) |
Table 3.
RA and RV strain indices presented as median (IQR). Exact significance calculated using a two-sided Mann-Whitney U test.
Table 3.
RA and RV strain indices presented as median (IQR). Exact significance calculated using a two-sided Mann-Whitney U test.
| MYH6 Variant (N = 19) | Control (N = 37) | p-Value | |
|---|---|---|---|
| RA GLS (%) | 18.2 (15.0, 24.1) | 22.2 (14.9, 24.9) | 0.263 |
| RA AScd (%) | 26.4 (20.7, 31.2) | 27.0 (19.7, 34.4) | 0.810 |
| RA ASct (%) | −1.41 (−2.13, −0.25) | −3.53 (−5.53, −1.28) | 0.008 ** |
| RA ASr (%) | 28.5 (21.2, 33.2) | 31.0 (24.7, 37.5) | 0.302 |
| RA ASRr (%/s) | 1.06 (0.78, 1.43) | 1.23 (1.05, 1.55) | 0.096 |
| RA ASRct (%/s) | −1.26 (−1.55, −0.99) | −1.40 (−1.71, −1.13) | 0.198 |
| RV GLS (%) | −12.5 (−15.0, −11.3) | −12.5 (−14.4, −10.7) | 0.683 |
| RV VSs (%) | −14.3 (−16.3, −12.9) | −14.6 (−17.4, −11.4) | 0.201 |
| RV VSRs (%/s) | −0.89 (−0.99, −0.77) | −0.78 (−1.07, −0.66) | 0.127 |
| RV VSRed (%/s) | 28.5 (21.2, 33.2) | 31.0 (24.7, 37.5) | 0.302 |
| Heart rate (bpm) | 145 (138, 157) | 154 (142, 162) | 0.283 |
Table 4.
Correlations of RA and RV strain indices with heart rate, presented with two-sided significance.
Table 4.
Correlations of RA and RV strain indices with heart rate, presented with two-sided significance.
| MYH6 Variant | Control | |||
|---|---|---|---|---|
| Pearson Correlation (R) | p-Value | Pearson Correlation (R) | p-Value | |
| RA GLS (%) | 0.268 | 0.267 | −0.044 | 0.796 |
| RA AScd (%) | 0.469 | 0.043 * | −0.176 | 0.297 |
| RA ASct (%) | −0.148 | 0.546 | −0.220 | 0.190 |
| RA ASr (%) | 0.499 | 0.029 * | −0.102 | 0.547 |
| RA ASRr (%/s) | 0.368 | 0.121 | −0.047 | 0.783 |
| RA ASRct (%/s) | −0.235 | 0.332 | 0.274 | 0.100 |
| RV GLS (%) | −0.001 | 0.997 | 0.325 | 0.050 * |
| RV VSs (%) | −0.069 | 0.779 | 0.419 | 0.010 * |
| RV VSRs (%) | −0.127 | 0.604 | 0.410 | 0.012 * |
| RV VSRed (%) | −0.100 | 0.685 | −0.265 | 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Source link
Melissa Quintanilla Anfinson www.mdpi.com