1. Introduction
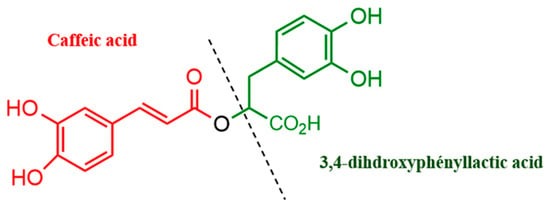
Bioactive compounds from natural products have attracted increasing interest due to their chemopreventive and chemotherapeutic potential for treating and preventing various diseases. Rosmarinic acid (RA) is an ester of caffeic acid (3,4-dihyroxcynnamic acid) and 3,4-dihdroxyphenyllactic acid (
Figure 1) that exhibits antioxidant [
1], anti-inflammatory [
2], and antimicrobial properties [
3] and is widely used in the pharmaceutical, cosmetic, and food industries [
4]. This phenolic compound with the molecular formula C
18H
16O
8, was first isolated by Scarpati and Oriente in 1958 from
Rosmarinus officinalis L. [
5].
Plants belonging to the Boraginaceae family, subfamily Nepetoideae (Lamiaceae or Labiaceae), such as rosemary (
Rosmarinus officinalis), sage (
Salvia officinalis), thyme (
Thymus vulgaris), oregano (
Origanum vulgare), and lemon balm (
Melissa officinalis), are a main source of RA. Previous studies have demonstrated the pharmacological effects of RA, including hepatoprotective activity [
6], antiallergic activity [
7], anti-ageing activity [
8], antidepressant potential [
9], cardioprotective activity [
10], and anti-cancer activity [
11]. Antidiabetic activity has been demonstrated [
12], revealing the inhibitory effect of a rosemary extract enriched with RA on the enzyme dipeptidyl peptidase IV (DPP-IV). Inhibiting this enzyme helps reduce hyperglycaemia and haemoglobin A1c levels. The authors also showed that RA has a high affinity for DPP-IV. This interaction was confirmed by a negative binding energy of −7.8 kcal/mol.
This phenolic acid is gaining increasing attention in the pharmaceutical industry due to its cytotoxic potential against various cancer cell lines. Encalada et al. demonstrated that RA, extracted from Melissa officinalis, exhibited significant cytotoxicity against human colon cancer cells (HCT-116) at a concentration of 1 mg/mL after 24 h [
13]. Additionally, rosemary extract rich in RA has shown notable cytotoxic effects against human osteosarcoma cells (MG-63) at concentrations exceeding 300 µg/mL [
14]. Due to its bioactive properties, RA has also found applications in the nutraceutical industry, particularly in the formulation of solid lipid nanoparticles using Witepsol waxes [
15]. Its potential as a functional food ingredient has been highlighted by its positive influence on gut microbiome growth when incorporated into solid lipid nanoparticles [
16].
With the growing demand for RA and concerns about the adverse effects of synthetic compounds on human health, the food industry is increasingly being pushed to adopt natural alternatives. Plant extracts, known for their many benefits, are increasingly used in food formulations to replace, partially or totally, synthetic additives [
17,
18]. However, to meet this increased demand, it is essential that extraction methods be developed that are both efficient and sustainable.
Many studies have been carried out on extracting RA using conventional methods, mainly based on the use of organic solvents. These techniques include maceration [
19], Soxhlet extraction [
20], and extraction by reflux heating [
21]. These methods are based on several key steps: the penetration of the solvent into the plant cells, the solubilisation of the phytochemicals in the plant matrix, and, finally, the diffusion of the phytochemical-enriched solvent out of the plant cells [
22]. However, these methods are often associated with limited yields, thermal degradation of sensitive compounds, and excessive use of organic solvents.
In this regard, innovative extraction methods such as scCO2 extraction, pressurised liquid extraction, and enzyme-assisted extraction have been considered as alternatives for RA extraction. In contrast, carbon dioxide in the supercritical state has proved to be a more effective alternative to conventional solvents. In the supercritical state, CO2 has properties that are the intermediate between those of a gas and a liquid, making it possible to penetrate plant matrices with high efficiency while selectively solubilising bioactive compounds. This technique offers significant advantages, such as the preservation of temperature-sensitive compounds, the absence of toxic residues, and a reduced environmental impact, in addition to allowing precise control of extraction conditions to maximise yields.
scCO
2 is recognised for its ability to efficiently extract compounds due to its solvent properties, which can be adjusted based on pressure and temperature conditions. By mod-ifying these parameters, it is possible to control the selectivity of scCO
2, particularly for the extraction of bioactive compounds [
23]. However, scCO
2 is more suitable for extracting apolar compounds. The addition of a co-solvent (modifier) enhances the solubility of polar compounds. Indeed, the dipole-dipole interactions and hydrogen bonds formed between the solute and the modifier significantly increase the solubility of the solute [
24]. Among the co-solvents commonly used, ethanol is characterised by its polarity, due to the presence of a hydroxyl group. It is widely used in the food industry, as it is less harmful than other organic solvents [
25]. The study conducted by Abdul Aziz et al. [
23] highlighted that the extraction of Orthosiphon stamineus leaves using scCO
2 with ethanol as a co-solvent resulted in a higher concentration of RA compared to other compounds extracted.
Due to RA’s high polarity, a co-solvent is necessary to increase its concentration, thus achieving levels comparable to those of conventional methods. A previous study [
26] found that rosemary extracts from scCO
2 extraction demonstrated a higher antiproliferative effect than those obtained from solvent due to their richness in bioactive compounds.
The RA content in plants rarely exceeds 1% of the dry weight [
27] and varies according to plant physiology, growth and development stages, geographical and environmental conditions, and pre-and post-harvest processes. In response to the growing demand for high-volume production, it is crucial to optimise the extraction parameters according to the matrix used to ensure high yields.
One of the main challenges of the extraction process is that a significant amount of RA remains trapped in the rosemary residues, resulting in a limited overall yield. Similarly, RA yield in scCO
2 extraction can vary depending on factors such as pressure, temperature, extraction time, and use of co-solvents. Existing studies do not provide precise data on the yield of RA for scCO
2 extraction, although they offer an overview of the overall yield of bioactive compounds [
28,
29,
30]. On the other hand, although the Soxhlet method effectively extracts bioactive compounds from
Rosmarinus officinalis L., it has significant limitations for extracting RA [
31]. In this context, relying solely on traditional methods, and even on innovative ones, does not allow us to exploit rosemary’s RA potential.
This study aims to optimise the conditions of RA extraction from Rosmarinus officinalis L. using supercritical scCO2 with ethanol as a co-solvent. The key factors of pressure, temperature, and co-solvent percentage were optimised using the Box–Behnken experimental design to find the best conditions for maximising RA content. In addition, a CO2-Soxhlet coupling method was employed to further improve extraction yield. This coupling, which combined the advantages of scCO2 extraction with the exhaustive extraction capabilities of the Soxhlet technique, represents an original feature of this work to improve RA yield and extract purity.
2. Materials and Methods
2.1. Chemicals
Folin Ciocalteu reagent, Trolox, and Na2CO3 were purchased from Sigma-Aldrich (Steinheim, Germany), with rosmarinic acid (purity > 97%) from Thermo Fisher Scientific (Kandel, Germany), carnosic acid (purity ≥ 90%) from Extrasynthese (Genay, France), and carnosol from MedChemExpress (Monmouth Junction, NJ, USA). The CO2 (purity 99.99%) was provided by Messer France. The analytical-grade solvents used were supplied by CARLO ERBA reagents (Val de Reuil, France).
2.2. Optimising scCO2 Extraction
2.2.1. Plant Material and Extraction Methods
Rosemary was harvested in May 2022 in the Beni-Chebal region, located in the province of Taourirt (34.172031, −2.722161), in an arid bioclimatic zone in eastern Morocco. The rosemary leaves, manually separated from the stems and dried in the shade, were finely ground using an electric grinder (Silver Crest). The resulting powder was then sieved to isolate a specific particle size fraction, with a particle diameter between 0.2 and 1 mm, used in this study.
The extractions were carried out using a supercritical fluid extraction system (SFE-210057-SY10-A, EXTRATEX, France) (
Figure S1 in Supplementary Materials). This device includes a 100 mL extractor placed in a temperature-controlled oven, a separate unit, and a recycling system. The system is equipped with a CO
2 pump to obtain the desired pressure and a co-solvent pump to adjust the polarity of the CO
2. A cylinder supplies the CO
2 at a pressure of over 50 bar.
A mass of 15 g of rosemary leaf powder was placed in the extractor. Extractions were carried out at temperatures ranging from 40 to 80 °C and under pressures between 150 and 350 bar. The CO2 flow rate was set at 15 g/min, with a static state maintained until the desired pressure was reached. Ethanol, used as a co-solvent, was used to improve the solubility of the RA, with a flow rate of between 5% and 15%, based on weight. The total extraction time was 3 h.
2.2.2. Response Surface Methodology
A Box–Behnken experimental design was used to investigate the impact of three scCO2 process factors—temperature, pressure, and co-solvent percentage—on total extraction yield and RA content. The experimental design was created using the “Design Expert” software (Version 13, Stat-Ease), and the data were analysed using analysis of variance (ANOVA), setting a significance level of 0.05. R-squared, adjusted R-squared, and p-values, alongside a lack-of-fit test, were used to assess the model’s suitability for the experimental data.
Table 1 shows the levels of the three factors studied: temperature (40, 60, and 80 °C), pressure (150, 250, and 350 bar), and the percentage of co-solvent (5, 10, and 15% ethanol). The results, i.e., the total extraction yield and RA content, were obtained from 15 experimental runs, including 3 central points.
The extraction conditions were chosen based on preliminary experiments and data published in the literature. The experimental design method aims to establish the relationships between factors (variables, X
i) and responses (results, Y) to define an a priori polynomial mathematical model linking the responses to the factors. The mathematical relationship between the variables and the responses can be approximated by applying the following polynomial Equation (1):
where y is the measured response, β0 is a constant value, βi is the linear coefficient, βii is the quadratic coefficient, and βij is the interaction coefficient, while Xi and Xj are independent variables.
2.3. Soxhlet Extraction
The Soxhlet extraction method was used as a reference method. A quantity of 15 g of rosemary leaf powder was placed in a cellulose extraction cartridge and then extracted with ethanol for 6 h. The residue of the rosemary powder, previously extracted by scCO2 under the conditions of 150 bar, 80 °C, and 10% ethanol, was then subjected to a second extraction with Soxhlet, again with ethanol and for 6 h, to maximise the RA content.
2.4. Determining the RA Content
Phenolic content was determined using ultrahigh performance liquid chromatography (UPLC) from Waters Acquity (Waters, Milford, MA, USA) equipped with an autosampler, a quaternary pump, a diode array detector, and a column furnace. Separation was performed on a C18 column (2.1 × 100 mm, 1.7 μm, Waters Acquity). The mobile phase was a mixture of (A) acetonitrile with 0.1% formic acid (v/v) and (B) water with 0.1% formic acid, at a flow rate of 0.39 mL/min under the following solvent B gradient conditions: 80% (0 min), 40% (2.55 min), 25% (4.25 min), 25% (5.10 min), and 80% (7 min). The column was maintained at 35 °C, with an injection volume of 1 μL, and the detection wavelength was 325 nm for RA and 280 nm for carnosic acid and carnosol.
The rosmarinic acid (RA, y = 12,069x + 793.9; R
2 = 0.999), carnosic acid (CA, y = 777.32x − 1024.8; R
2 = 0.98), and carnosol (CAR, 538.21x − 375; R
2 = 0.95) contents were expressed as the ratio of the quantified amounts of each compound by UPLC to the mass of dry rosemary, as shown in the following Equation (2):
The compound weight, denoted as “x”, is determined from the calibration curve, which correlates the peak area of “y” with “x”.
2.5. Evaluating Antioxidant Activity
The antioxidant power of the extracts was determined using the DPPH (1,1-diphenyl-2-picrylhydrazil) free radical scavenging method according to the protocol described by [
32] with a few modifications. Absorbance was measured at 517 nm. All assays were performed in triplicate. The percentage inhibition (PI) of DPPH was determined using the following Equation (3) [
33]:
where PI is the percentage of inhibition, A0 is the optics density of the free radical (DPPH) solutions in the absence of the extract (negative control), and A is the absorbance of the free radical (DPPH) solution in the presence of the extract.
The anti-free radical power was expressed by the IC50 value calculated from the regression curves. IC50 corresponds to the inhibitory concentration required to reduce 50% of free radicals. A lower value of IC50 indicated greater anti-free radical activity.
2.6. Scanning Electron Microscopy
The surface of the rosemary powder was analysed before and after supercritical fluid extraction using an FEI Quanta-250 environmental scanning analytical electron microscope.