1. Introduction
Microbial keratitis has an annual incidence of approx. 1.5–2 million cases in developing countries [
1,
2]. It leads to corneal opacity, which is one of the main causes of corneal blindness worldwide [
1,
2]. The most common risk factor in approximately 70% of all cases is the wearing of contact lenses [
3]. Bacterial infections of the corneal tissue account for 90% of all microbial keratitis cases [
3,
4],
Aspergillus spp.,
Fusarium spp. and
Candida albicans [
5], as well as viruses such as herpes simplex virus [
1], are further pathogens.
Acanthamoeba keratitis is rare but particularly worrying, as it is associated with severe eye damage [
6].
Acanthamoeba spp. are protozoans that are naturally found in dust, soil, and water, and the contamination of contact lenses usually occurs through unhygienic cleaning methods [
1]. Infections are difficult to diagnose and to treat. Due to the relative rarity of
Acanthamoeba keratitis compared to other causes of keratitis (bacterial, fungal, viral), it is often misdiagnosed, especially in the early stages of the disease [
7]. If not diagnosed and left untreated,
Acanthamoeba keratitis can lead to irreversible vision loss; if recognised early, it can be cured by treatment with a combination of biguanide and aromatic diadine antibiotics given for several months [
6].
The survival, virulence, and antibiotic resistance of corneal
Acanthamoeba can be enhanced in the presence of endosymbiotic bacteria [
8,
9,
10,
11], which persist intracellularly and are transmitted within the protozoan [
12]. Among these endosymbionts are well-known pathogens, such as
Legionella pneumophila,
Coxiella burnetii,
Pseudomonas aeruginosa,
Helicobacter pylori,
Cryptococcus neoformans, and
Chlamydia trachomatis [
13,
14], as well as less-known genera of the order Holosporales, such as Candidatus
Caedibacter and
Paracaedibacter, which have emerged as potential pathogens in corneal infections [
8,
9,
10,
11].
Acanthamoeba can also affect endosymbiont virulence and invasiveness by protecting it from antibiotic treatment and providing good growing conditions. In addition, the interaction between host and endosymbiont may exacerbate the course of keratitis due to the presence of pro-inflammatory bacterial compounds [
15].
For the diagnosis of microbial keratitis, a corneal culture remains the routine standard procedure; however, this approach is impaired by long diagnostic turnaround times and low sensitivity [
1,
2]. Confocal corneal microscopy is significantly faster compared to culture but with little differentiation between bacterial and fungal keratitis [
16]. Molecular genetic methods, like species-specific qPCR or broad-range qPCR-based Sanger sequencing, have already shown convincing results in the diagnosis of keratitis [
2]. Recent studies demonstrated PCR as a suitable tool for the diagnosis of
Acanthamoeba keratitis, with a sensitivity of 70–100% and a specificity of 90–100% [
3,
17,
18,
19,
20,
21]. The limitation of PCR is the diagnostic restriction of the primers used; the more specific the primers are, the greater the risk that pathogen variants will be missed [
2].
In recent years, several attempts have been made to define the ocular microbiome, but this has not yet been well-characterised [
22]. While, in the past, culture-based methods were mainly used to analyse the ocular microbiome, through which only a few microorganisms were identifiable, more recently, the focus has been on 16S rDNA-based next generation sequencing (NGS) methods, which, in contrast to culture-based methods, has revealed a rich bacterial ocular microbiome, mainly consisting of staphylococci, streptococci, propionibacteria, and micrococci [
22,
23,
24].
Metagenomics/shotgun whole-genome sequencing (WGS), would enable the detection of unexpected pathogens by identifying the entire microbial DNA in a sample. This means that all bacteria, fungi, DNA viruses, and parasites can be detected in a single analysis. Unfortunately, WGS has not yet become established in the routine diagnostics of microbial keratitis because of the high costs and long processing times, although its potential to detect the microbiome including pathogens and their endosymbionts could revolutionise our understanding of microbial interactions in ocular infections [
2].
In this pilot study, we take the first steps to validate a diagnostic application for WGS for pathogen detection in Acanthamoeba-associated keratitis and compare WGS with the routine standard methods of confocal corneal microscopy and PCR.
3. Results
3.1. Case Report
A 46-year-old male patient presented to the Department of Ophthalmology at the University Clinic of Duesseldorf complaining of eye pain for three months with significant worsening over the last 3 days, with reddening of the left eye, epiphora, photophobia, blepharospasm, and significant visual deterioration. He had been experiencing eye pain for three months and had been using soft contact lenses up to that point.
The examination of the right eye showed normal age-appropriate findings, whereas the left eye showed a clear conjunctival injection with chemosis and corneal decompensation. In the centre of the cornea was a dense, large ring infiltrate with a surface defect, which made a more precise assessment of the anterior eye chamber impossible. A hypopyon was not detected. Sonography showed no evidence of vitreous involvement.
An eye swab with subsequent routine diagnostic in-house qPCR for the detection of Acanthamoeba and a bacterial pan-PCR were positive and revealed Acanthamoeba spp. The patient was hospitalised, and inpatient therapy was performed with propamidine isoethionate, polyhexanide, and atropine eye drops for Acanthamoeba keratitis. As there was no improvement after one day, topical voriconazole was added. Confocal corneal microscopy showed the characteristic cysts compatible with acanthamoebae.
During the clinical stay, the irritation of the left eye improved. The patient reported a subjective decrease in pain and blepharospasm. The infiltrate became more clearly demarcated, and the epithelial defect showed a regressive tendency. As the corneal oedema decreased, endothelial precipitates and irritation of the anterior chamber became progressive. As adjuvant therapy, corneal crosslinking (riboflavin eye drops in combination with UVA irradiation) was performed. As the clinical presentation continued to improve, a penetrating keratoplasty à chaud was performed as an inpatient procedure for germ reduction. A corneal biopsy was sent to the Institute of Medical Microbiology and Hospital Hygiene. Postoperatively, there were no complications after keratoplasty with an improvement in visual acuity. The patient was discharged three days after keratoplasty with significantly reduced therapy with polyhexanide and dexamethasone and no evidence of a recurrence of the infection. Close monitoring was carried out up to four months postoperatively, at which time no further pathology was observed.
3.2. Microbiological Diagnostics
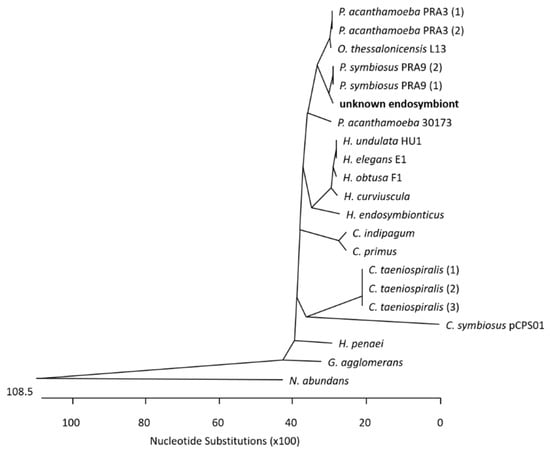
At the Institute of Medical Microbiology and Hospital Hygiene, the genomic DNA of the corneal biopsy was positive for genus-specific Acanthamoeba-PCR. A pan-bacterial 16S rDNA PCR was performed, and Sanger sequencing of the 16S rDNA amplicon (V1–V3 region) led to the identification of a bacterium belonging to the order Holosporales, members of which are known endosymbionts of Acanthamoeba spp. To narrow down the bacterial species, further bacterial Pan-PCRs were carried out amplifying and subsequently decoding 1.4 kb 16S rDNA region in total. A multiple-sequence alignment of the query to 16S rDNA homologue regions in known Holosporales genomes (see
Supplementary File S1, Sheet Holosporales Species) enabled the construction of a phylogenetic tree (see
Figure 1), which suggests that the patient’s endosymbiont belongs to Candidatus
Paracaedibacter symbiosus.
To validate the power of WGS as a one-step molecular genetic diagnostic approach, we sequenced the DNA sample from the keratoplasty biopsy using Oxford Nanopore Sequencing Technology (ONT). A bioinformatic analysis was performed using the k-mer-based approach of Kraken2. A total of 3,203,564 reads (99.92%) were classified as
Homo sapiens, 1683 reads (0.05%) were classified as
Acanthamoeba castellanii (strain Neff), 108 reads (<0.005%) were classified as
Arthrobacter spp. KBS0702, 35 reads (<0.005%) were classified as
Plasmodium vivax, and 32 reads (<0.005%) were classified as Candidatus
Paracaedibacter symbiosus (see
Table 1).
A Minimap2-based remapping of the reads to the respective genome sequences was used to verify the results (
Table 2). The identification of Candidatus Paracaedibacter symbiosus and Acanthamoeba castellanii was confirmed by the verification step. A total of 90.6% of the Candidatus Paracaedibacter symbiosus reads and 55% of the Acanthamoeba castellanii reads were mapped to the respective reference genomes.
However, 49% of the Acanthamoeba reads were mapped to Homo sapiens, indicating that some of the Homo sapiens reads had been incorrectly classified as Acanthamoeba. Misclassification by Kraken2 became also obvious for Arthrobacter spp. and Plasmodium vivax reads. In particular, the supposed Arthrobacter spp. reads were all mapped to Homo sapiens, and the Plasmodium vivax reads were mapped back to both Homo sapiens (100%) and Plasmodium vivax (97.1%). This result suggests contamination of the reference genomes in the data bank (see discussion) and implies that a bioinformatic verification step of WGS data is mandatory.
For the genus Acanthamoeba, 23 different genotypes (type T1 to T23) had been identified, as defined by differences in the 18S rDNA gene, with T4 as the most frequently identified genotype in AK cases [
30]. To determine which genotype the detected Acanthamoeba was assigned to, we sequenced the 180 bp 18S rDNA-based Acanthamoeba-PCR product of the routine diagnostics, which covered the 5′ end of the JDP1–JDP2 targeted region (that is commonly used for genotyping) [
31]. Additionally, we mapped the Nanopore sequence reads to the 18S rDNA gene of Acanthamoeba castellanii strain Neff and extracted the three overlapping reads. The consensus sequence of the PCR product and the Nanopore reads of 16,171 bp covered the whole genotyping region JPD1–JDP2 (see
Figure 2). A Blast analysis of this sequence was conducted. Of the possible 23 genotypes, only the T4 sequence was found.