1. Introduction
Masonry fired-clay brick is one of the earliest known building and construction materials, and its demand has grown in tandem with the world’s population [
1]. Global manufacturing produces approximately 1.5 trillion fired-clay bricks annually [
2]. An energy-intensive method is used worldwide to produce bricks at high temperatures. Burnt bricks release NO
x and CO
2 into the atmosphere due to their heavy reliance on fossil fuels. However, the depletion of clay resources might result from the use of clay as a raw material for brickmaking [
3]. This is why it is not ecologically advantageous to manufacture burned bricks from clay resources, despite the high energy demand and resource use involved. An economically and environmentally beneficial alternative might be to use garbage as a raw material for brick production [
4]. It encourages environmental sustainability in addition to reducing disposal costs at landfills [
5,
6]. Environmental issues will worsen as waste and industrial byproduct volumes increase, and disposal space becomes scarce [
7]. As a result, recycling materials and byproducts from solid waste management has become a preferred option over disposal [
8]. Reusing this industrial waste by converting it into fresh building materials is considered a practical solution to reduce environmental pollution problems [
9]. However, in order to recycle this waste, its environmental traits and behaviors must meet certain requirements and adhere to relevant environmental guidelines [
10].
Ever since the geopolymerization process was discovered by Davidovits [
11]. The production of several types of bricks using this novel substance is beginning to pose a serious threat to conventional cement-based civil building methods [
12,
13,
14]. These materials include asphalt, roofing slates, concrete, and aircraft pavement [
15,
16,
17,
18,
19,
20,
21,
22,
23]. The primary step in the creation of geopolymer structures is the reaction of an alkaline activator with a binding material. Fly ash, or metakaolin, which is created by heating kaolin over 600 °C, is often used to make the former material. The binder group includes iron slag, red mud, and silica fume [
24,
25,
26,
27,
28,
29]. Recently, researchers have used other binding materials like leftover clay bricks or ceramic tile dust [
30,
31]. On the other hand, alkaline activators typically combine sodium silicate and sodium hydroxide in varying amounts to maintain a specific level of total sodium oxide. Zhuang et al. [
32] and Degirmenci [
33] have reported on the relationship between a stronger final geopolymer and a larger ratio of silicate to caustic soda. Caustic soda, the primary activator, typically combines with additional activators like slaked lime [
34,
35].
The low alumina and calcium contents of the waste foundry sand necessitated the addition of various amounts of fly ash and electric arc furnace slag to improve the alumina and calcium ratios and, consequently, the compressive strength. This was the main material composition used to fabricate geopolymer bricks illuminated by Suchanya Apithanyasai et al. [
8] for pavement applications. In a similar vein, a secondary supply of alumina was used to create a geopolymer based on rice husk ash with enhanced characteristics [
36,
37,
38,
39,
40,
41]. Ehsan Mohseni et al. [
42] nano-Al
2O
3 is added to rice husk ash to make up for the absence of Al
2O
3 in the rice husk ash (RHA) and produces a rice-husk-ash-based geopolymer. Producing a geopolymer gel, calcium silicate hydrate, calcium aluminosilicate hydrate, or sodium aluminosilicate hydrate in the alkali-activated matrix further enhanced the mechanical characteristics [
43]. So, alumina must be added to low-alumina materials [
44]. Similar to glass or ceramic foams, which are made at temperatures exceeding 900 °C, geopolymer foams may be produced at low temperatures (below 100 °C) [
45,
46]. High-temperature applications, such as wall panels, thermal insulation, and fire-resistant coatings, utilize geopolymer foams [
45,
47,
48,
49]. Most of these foams are lightweight, making them suitable for applications requiring thermoacoustic insulation and fire resistance [
45,
50]. They depend on the essential use of foaming agents as materials that generate pores.
Ibrahim et al. [
51] produced fly-ash-based geopolymer bricks using sodium silicate and caustic soda as an activating solution, along with an unidentified foaming component at levels ranging from 5 to 10%. Their samples revealed densities between 1400 and 1500 kg/m
3, which were associated with compressive strengths ranging from 5 to 10 MPa, depending on the amount of foaming agent used. Despite this, these densities are too high for insulating components. Risdanareni et al. [
52] also obtained similar results using Styrofoam as a pore-generating material at levels as high as 0.9%. On the other hand, Roviello et al. [
53] created hybrid geopolymer-based foams that had lower densities (250–850 kg/m
3) but great mechanical properties, fire resistance, and low thermal conductivity. The manufacture of geopolymers also used hydrogen peroxide as a foaming agent to create porosity [
54,
55,
56,
57]. Ahmed et al. [
58] reported on the fabrication of thermally insulating geopolymer bricks using ferrosilicon slag and alumina waste. They achieved the maximum compressive strength at a concentration of 8 M NaOH. Furthermore, the inclusion of alumina waste decreased the heat conductivity of the resultant geopolymer bricks. El-Naggar et al. created geopolymer-insulating bricks using waste materials that had a bulk density of around 1000 kg/m
3, low heat conductivity, and good mechanical strength [
59].
Exposure to aluminum dross waste is considered dangerous to human health and the environment, and since 2000, the EU has classified it as such. Specifically, discarded aluminum dross is considered to be carcinogenic as well as a source of skin irritation and corrosion. The non-metallic product (NMP) component of aluminum dross waste also functions as a sensitizer and irritant when it comes into extended or regular contact with the skin or mucous membranes [
60]. Dust is created during the processing of aluminum dross waste, and inhaling or consuming it presents a major danger. Moreover, the active contaminants in aluminum dross waste react rapidly with moisture to produce gasses that are poisonous, explosive, and offensively odorous [
61]. In the past, landfills disposed of about 95% of the industrially generated aluminum dross waste annually [
62]. However, due to the constant aluminum production, the remaining 5% has been steadily increasing. To make geopolymer concrete, Migunthanna et al. employed fly ash, slag in the precursor, and one-part binders made from leftover clay brick powder [
63]. Another study by Tazune et al. [
64] looked at how different Fe
2O
3/SiO
2 molar ratios in Fe-silica affected the properties of geopolymer materials made from metakaolin and waste-fired-clay brick.
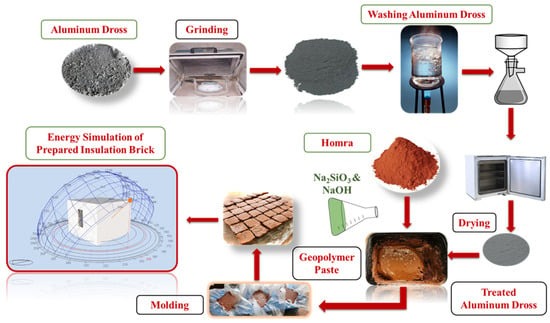
According to prior research, no study has employed aluminum melting waste dross to create lightweight geopolymer concrete or geopolymer bricks that insulate against heat. Thus, the significance of this experimental study lies in its potential use in the geopolymer brick industry to produce lightweight thermal insulation bricks from leftover aluminum dross. This work will open doors and encourage the construction industry to employ aluminum dross from industrial waste in geopolymer. This study suggests using wasted bricks as a solid starter and a solution of sodium hydroxide and sodium silicate as an alkaline activator to make inexpensive low-density insulating geopolymer bricks. Porosity forms when waste aluminum dross from various factories melts and combines with an alkaline activator. The importance of this study lies in the fabrication of a lightweight, room-temperature geopolymer brick that can replace burned bricks.