Rheumatoid arthritis (RA) is an autoimmune disease characterized by tenosynovitis, leading to cartilage destruction and bone erosion, affecting multiple joints bilaterally [1]. RA significantly diminishes patients’ quality of life and imposes a substantial economic burden on both patients’ families and society. Severe complications, such as rheumatoid nodules, rheumatoid vasculitis, and cardiovascular diseases, which are also the primary causes of death, can arise in patients with RA [2]. Currently, the etiology of rheumatoid arthritis remains unclear. While immunosuppressive therapy based on autoimmune disorders can alleviate symptoms and delay the progression of rheumatoid arthritis, it cannot provide a cure [3].
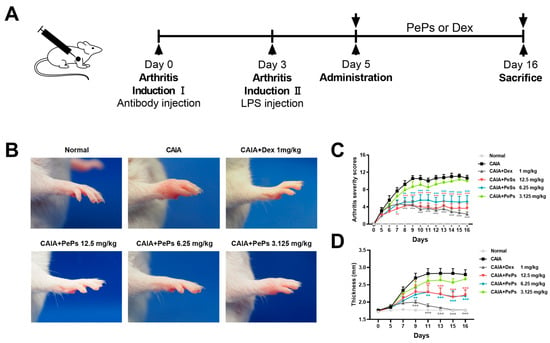
Various natural products have been consistently studied for their potential in treating rheumatoid arthritis [4]. Cortex periplocae, also known as ‘Xiangjiapi’, is a traditional Chinese herbal medicine, derived from the root bark of Periploca sepium Bunge [5]. It has been extensively used as a folk medicine agent for treating rheumatoid arthritis in China [5,6]. As reported, pregnane glycosides, the primary components of Cortex periplocae, exhibit promising anti-inflammatory activity [7]. We prepared Periploca sepium periplosides (PePs) as a pregnane glycoside fraction free of cardiac glycosides, extracted from the root bark of Cortex periplocae. PePs consist of eight main pregnane glycosides: periplocosides A (PSA), C, D, E, K, O, Q, and R. In our team’s previous research, we discovered that PSA, which is a major component of PePs, exhibits superior immunosuppressive activity both in vitro and in vivo [8]. Furthermore, we established collagen-induced arthritis (CIA) and rat adjuvant-induced arthritis (AIA) models to evaluate the therapeutic efficacy of PePs, and the results demonstrated their remarkable anti-arthritis effects [6]. AIA and CIA are acknowledged to rely on T-cell activity, as they are induced through antigen-specific T-cell activation and migration [9]. Conversely, collagen antibody-induced arthritis (CAIA) is characterized by its independence from either T cells or B cells, as its induction is attributed to the infiltration of neutrophils and macrophages into the joints. In CAIA models, joint damage is initiated by targeting collagen type II, a key structural component of cartilage, through a cocktail of monoclonal antibodies. These antibodies bind to specific regions of the collagen, triggering an inflammatory response that leads to joint tissue degradation. To amplify this inflammatory cascade and accelerate joint swelling and damage, lipopolysaccharide (LPS) is administered after the antibody treatment. This combination of immune activation and inflammation contributes to the progressive destruction of the joint structure [10]. Hence, further investigation is warranted to fully explore the potential therapeutic effects of PePs on CAIA.
The utilization of the CAIA model in mice offers a direct and effective method for exploring the fundamental mechanisms implicated in rheumatoid arthritis pathogenesis, as well as facilitating the screening of potential therapeutic agents [11]. In light of our previous research findings, the primary objective of this study is to evaluate the therapeutic efficacy of PePs in the treatment of CAIA through the modulation of macrophage polarization in an in vivo setting, thus building upon our existing findings. Furthermore, we seek to simulate the process of polarization using RAW264.7 cells in vitro, aiming to investigate the specific impact of PePs on macrophage polarization.
Source link
Que Wang www.mdpi.com