1. Introduction
Osteoarthritis (OA), a medical condition commonly associated with aging, involves the degeneration of joint structures, leading to pain and functional limitations [
1,
2,
3,
4]. Acromioclavicular (AC) joint OA is the most common source of shoulder pain in middle-aged individuals, especially with regular overhead and cross-body tasks [
5,
6]. Further studies found that 54–57% of patients who are elderly show signs of degenerative arthritis at the AC joint [
7,
8]. Despite its prevalence, AC joint OA diagnostics face significant limitations, including variability in the sensitivity and specificity of physical tests and the inability of standard imaging techniques to accurately capture early degenerative changes or functional impairments [
9,
10].
Healthcare providers and researchers continue to study AC joint OA’s pathogenesis and effective clinical management to understand its pathogenesis and clinical management. Various tests have been used to detect OA of the AC joint, including imaging and physical examinations, but diagnostic accuracy remains challenging. Clinicians perform physical examinations by placing and moving the shoulder to induce pain at the AC joint and detect OA. The cross-body adduction stress test, the acromioclavicular resisted-extension test, the Buchberger test, the active compression test, and the Paxions test are pain-inducing physical examination techniques commonly used to detect pathology in the AC joint [
7,
8,
11,
12,
13,
14,
15]. However, these tests often lack consistency, with false negatives or misdiagnoses frequently reported. For instance, the cross-body adduction (CBA) test is a primary clinical diagnostic procedure for OA in the AC joint [
16]. Although the CBA test is commonly used in clinical settings, inconsistencies have been observed in its test results. In particular, some patients who test negative for pain with the CBA test later test positive and are diagnosed with AC joint OA through imaging or arthroscopic procedure. These limitations highlight the need for enhanced diagnostic tools integrating biomechanical insights to improve clinical reliability.
A recent study by Bethany et al. [
17] proposed a new clinical test, the hand-behind-the-back (HBB) test, as a potentially superior diagnostic tool for AC joint OA. It hypothesizes it could offer a better physical diagnosis for AC joint OA in clinical trials with greater sensitivity. The HBB test resulted in more than a five-fold reduction in the AC joint articulating surface compared to the CBA test, offering a unique mechanical perspective [
17]. Given these findings, our study uses musculoskeletal modeling software to focus on understanding the muscle force requirements during the CBA and HBB tests. The decision to prioritize the CBA and HBB tests over other diagnostic methods stems from their distinct mechanical challenges, frequent clinical use, and potential to shed light on specific AC joint mechanics.
The objective of this study is to predict muscle forces during the CBA and HBB tests using two musculoskeletal modeling software programs, OpenSim and AnyBody Modeling SystemTM, and to evaluate the outcomes between the two. This research addresses gaps in AC joint biomechanics by providing detailed muscle force predictions and identifying variations between the software platforms regarding force magnitude and activation patterns. A deeper understanding of these forces can enhance diagnostic accuracy, inform rehabilitation strategies targeting specific muscle groups, and underline the role of accessory muscles in improving patient outcomes.
2. Materials and Methods
For this study, we first sought a model that accurately reflects the anthropometry and muscle force-generating capacity of a 50th percentile adult male to ensure consistency between the software platforms, even with the generic models. Further, we wanted to ensure it includes the three degrees of freedom (DOF) at the GH joint to perform flexion/extension, abduction/adduction, and internal/external rotation. Similarly, two DOFs at the elbow joint perform flexion/extension and pronation/supination rotation as required for the CBA and HBB tests. The Stanford VA upper limb model [
18], model of the scapulothoracic joint [
19], dynamics arm simulator [
20], and upper extremity dynamic model (UEDM) [
21,
22] are some commonly used shoulder models from OpenSim. This study uses the UEDM from OpenSim (Version 4.4, developed by the OpenSim team at Stanford University, Stanford, CA, USA,
https://simtk.org/projects/opensim, accessed on 15 May 2024) because it meets the criteria set for the model requirement as mentioned above. Plus, it can perform kinetics analysis, is easy to use without requiring external scripting or modifications and has been validated by the author for a wide range of motion [
21,
22]. Unlike OpenSim, the AnyBody Modeling System
TM typically includes one primary shoulder model, the shoulder arm model, based on a 50th percentile European male, and provides a detailed representation of the shoulder joint. This work uses the shoulder arm model from AnyBody Modeling System
TM (version 7.1.0.5957 developed by AnyBody Technology A/S, Aalborg, Denmark,
https://www.anybodytech.com, accessed on 5 July 2024) [
23].
The selection of OpenSim and AnyBody Modeling System™ was driven by their advanced dynamic modeling capabilities and applicability to perform upper extremity biomechanical analyses. To expand on the selected models, both include the upper extremity bones: thorax, clavicle, scapula, humerus, ulna, radius, and hand. Further, these segments form an articulation surface or joint of the upper limb, enabling the upper extremity to achieve the desired motion for daily activities. The UEDM comprises fifty Hill-type muscle–tendon actuators representing 32 muscles that span the shoulder, elbow, forearm, and wrist with a single line of action. At the same time, AnyBody Modeling SystemTM shoulder models consist of 118 full-blown Hill-type muscle–tendon units with at least two lines of action to define their point of origin and insertion.
2.1. Kinematics Definition
The kinematics foundation for the CBA and HBB tests is based on the study by Bethany et al. [
17]. This study provides the foundation of joint angles required to achieve the final resting posture for the CBA and HBB test. Because of this particular reason of predefined joint angles and the static nature of the simulation, motion capture validation of the joint angles was not performed. Instead, it builds upon previously validated models and establishes a foundation for future experimental validation. However, kinematics on prior study were defined for models created in MacNeal–Schwendler Corporation (MSC) automated dynamic analysis of mechanical systems (ADAMS) (MSC Software Corporation, Newport Beach, CA, USA). The provided joint angles did not align with the more anatomically realistic models available in OpenSim and AnyBody Modeling System
TM. More specifically, the sternoclavicular joint was fixed, limiting the relative motion between the humerus and bones of the shoulder complex. The wrist and elbow were modeled as spherical joints, while anatomically, they are synovial ellipsoid joints [
17]. This work started with similar joint angles as per the MSC ADAMS model by Bethany et al. [
17] and then gradually adjusted the kinematics by trial and error in OpenSim and AnyBody Modeling System
TM models, respectively, to obtain the identical final resting posture for the CBA and HBB tests as obtained by Bethany et al. [
17]. Bethany et al. [
17] mentioned an eighteen-degree elbow superior rotation at the elbow joint for the CBA test. However, this was found to be anatomically incorrect for both platforms, as the elbow primarily allows flexion and extension, not superior. We adjusted the elbow kinematics to reflect better realistic motion, which is essential for preventing unnatural upper-arm movements and ensuring proper elbow mechanics. Similarly, the model by Bethany et al. [
17] proposed a fifteen-degree radial deviation of the wrist for the final posture of the CBA test. However, during our simulation, we found this wrist deviation unnecessary. The desired resting posture could be achieved through adjustments to the shoulder and elbow joints, resulting in a more anatomically accurate model without relying on wrist deviation.
For the HBB test, Bethany et al. [
17] described a ninety-degree wrist and a forty-degree elbow pronation, which seemed inconsistent with typical joint function since pronation occurs at the forearm (between the radius and ulna). We corrected this error through successive adjustments by applying elbow pronation, which arises at the forearm, resulting in a more realistic simulation of the HBB posture. After these adjustments, the GH joint angle was adjusted to obtain the identical resting postures for the CBA and HBB tests using the OpenSim and AnyBody Modeling System
TM. Adjusting all these joint angles, more anatomically correct joint angles required to achieve the final resting posture for the CBA and HBB tests were obtained in successive iterations. It was found that the CBA and HBB were completed in five steps, as discussed below, using the available degrees of freedom in respective models from OpenSim and AnyBody Modeling System
TM.
2.1.1. Cross-Body Adduction Test
The CBA test was completed in 5 steps, starting from an anatomical position, as listed in
Table 1.
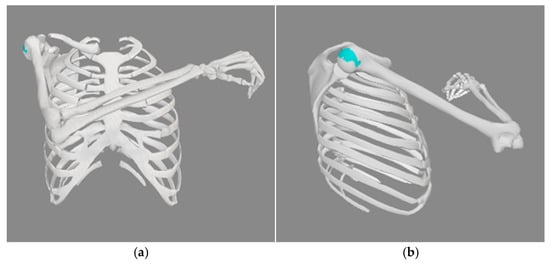
In OpenSim, the first step of a 90° internal rotation about the glenohumeral joint was achieved by setting the shoulder_rot coordinate to 90°. The second step was to perform a pronation of 20 degrees, which was done by setting the pro_sup coordinate to 20°. This will place the arm so that the thumb is facing forward. The third step was to have a shoulder flexion of 60°, which was done by first setting an elv_angle to 90° and shoulder_elv to 60°. Elv_angle sets the plane of motion and can change from −100° to 135°. Elv_angle of 0° means the frontal or coronal plane where the abduction/adduction motion occurs, and 90° means the sagittal plane where flexion/extension of the shoulder occurs. Suppose we increase the elv_angle from 90°. In that case, it will move the plane of motion towards the medial end of the body, and the arm movement occurs on that plane. At the same time, the negative value sets the plane of motion in the posterior direction of the body. The fourth step is 10° adduction, which was achieved by increasing the elv_angle by 10° from step three so that the arms will move toward the center. Finally, an elbow flexion of 90° is set to achieve the final resting position for the CBA in OpenSim, as shown in
Figure 1.
In the AnyBody Modeling System
TM, the joint angles are applied using the mannequin.any file, which takes both the joint positions and joint velocity as an input. Since this study focuses only on the static cases, the joint angles summarized in
Table 1 were changed in the mannequin.any file, using the corresponding joint names. Additionally, joint velocities were set to the default zero value to achieve the final resting posture for the CBA test. The only difference was in the adduction angle, which was set to 24° in the AnyBody Modeling System
TM to match the final resting position of the CBA test obtained from OpenSim, as shown in
Figure 2.
2.1.2. Hand-Behind-the-Back Test
The HBB test was also completed in 5 steps, starting from an initial position of selected generic models available in the OpenSim and AnyBody Modeling System
TM generic models, as listed in
Table 2.
In OpenSim, an internal rotation and pronation of 90° and 20° were first applied by changing the shoulder_rot and pro_sup coordinates within the graphical user interface (GUI). Then, for a 45° extension of the shoulder, the elv_angle is changed to −90°, corresponding to the sagittal plane. Finally, shoulder_elv is provided with 45° to obtain the shoulder extension. The next step was to supply a 10° adduction motion to push the arm towards the body. It was obtained by increasing the plane of elevation (elv_angle) to −100° so that the arm moves further towards the body. Finally, the elbow_flexion was set to 120° to obtain the final resting posture for the CBA test in OpenSim, as shown in
Figure 3.
In the AnyBody Modeling System
TM, similar rotation angles were applied within the mannequin file, as summarized in
Table 2, to achieve the final resting posture for the HBB test. The only notable difference was in shoulder extension, as the AnyBody Modeling System
TM did not require the change of plane; instead, a single variable GHFlexion was set to −45° to achieve this step. Finally, the elbow was flexed by 120° to accomplish the final resting position for the HBB, as shown in
Figure 4.
2.2. Simulation Procedure
In OpenSim, the standard simulation procedure was followed, as discussed in the papers by Delp et al. [
24] and Seth et al. [
25], to predict the muscle force. In the first step, a forward dynamics simulation for 1 s was performed to obtain the motion file (.mot file) for the CBA and HBB test by defining the kinematics presented in
Table 1 and
Table 2. In this step, coordinates were locked so they do not move; instead, they hold the set coordinate angle for the CBA and HBB test. So, the motion file will give us the joint angles that are just the constant value for each discrete time step for the entire duration of the simulation. The duration of the forward simulation, whether 0.1 s, 1 s, or 10 s, does not affect the results in this case, as the joint angles remain constant. A 1 s duration was chosen in this study to make sure we get enough time steps while minimizing computational time. Further, for static cases like this, the kinematic and kinetics results remain the same for each time step irrespective of simulation duration.
The resulting motion file was then used as input for static optimization (SO) to solve the muscle redundancy problem, minimizing the objective function as follows:
where is the number of muscles and is the muscle’s activation level at a discrete time step. Further, along with the motion file, reserve actuators were appended to each degree of freedom to provide any additional force required to obtain the desired kinematics of tests and to run the simulation successfully. Since this work only simulated the static postures of the CBA and HBB tests based on known kinematics or joint angles, scaling the models based on the subject-specific motion capture data was not required. However, this model has been validated against the subject-specific data in previous research [22], and their ability to represent typical upper extremity motions is well established. However, it is essential to acknowledge that this model is not validated using subject-specific data related to this study. Finally, the reserved actuators’ force predictions were checked to ensure they have no forces greater than 10 Newtons. This work predicted the muscle forces for the CBA and HBB test using OpenSim v4.4 [24,25].
In the AnyBody Modeling System
TM, an initial calibration was first run to calibrate the muscle parameters associated with the MuscleModel3E muscle model. Then, an inverse dynamics analysis was run to solve the muscle redundancy problem using a polynomial muscle recruitment criterion as follows [
23]:
where is an objective function, is the number of muscles, is the respective predicted muscle force in Newtons, and is isometric muscle strength in Newtons. This work predicted muscle force using AnyBody Modeling SystemTM v7.1.
4. Discussion
This study predicted muscle forces using two widely recognized biomechanical modeling software programs, OpenSim and AnyBody Modeling SystemTM, for two clinical tests for the AC joint OA: the CBA and the HBB test. The results obtained from this study highlighted observable agreements and discrepancies in muscle force predictions between the two software platforms. While the magnitude of the muscle force predictions differed between OpenSim and AnyBody Modeling SystemTM, the muscle activation patterns showed qualitative similarities. This presents the complexities associated with musculoskeletal modeling, its limitations, and its implications for clinical orthopedic practice and biomechanical research. Understanding the individual function of each muscle’s ability to strain the AC joint can reliably dictate its influence on a positive test result to identify AC joint OA. The deltoid has three heads, anterior, middle, and posterior, that flex and medially rotate, abduct, or extend and laterally rotate the arm. The anterior head of the deltoid plays more of a role in the CBA test, while the posterior head plays more of a role in the HBB test to position the arm behind the patient. While the coracobrachialis, pectoralis major, and latissimus dorsi do not cross the AC joint, they are strong muscle forces in arm positioning and glenohumeral motion that aid in forces placed across the AC joint. Additional accessory muscles that do not directly cross the AC joint and assist in stabilizing the shoulder girdle or arm/forearm positioning during the clinic tests (CBA/HBB) include the pectoralis minor, brachialis, biceps short head, serratus, sternocleidomastoid, and triceps. Of note, the rotator cuff muscles (supraspinatus, infraspinatus, teres minor, and subscapularis) are shoulder-stabilizing muscles as well that allow rotation of the glenohumeral joint to assist in arm positioning. However, they do not directly cross the AC joint articulation.
The muscle force predictions by the AnyBody Modeling System
TM and OpenSim during the CBA test offer helpful insights into the muscles potentially involved in this motion. Notably, both software platforms showed a close match in predicting the involvement of the deltoid clavicular muscle, suggesting its important role in performing the CBA test. This finding aligns with studies on shoulder biomechanics, which underscore the deltoid clavicular muscle’s role in shoulder elevation, shoulder rotation, and shoulder adduction due to its anatomical origin crossing the AC joint [
26,
27,
28]. Clinically, the forces generated by the deltoid muscle are significant, as they correlate with stress across the AC joint, a factor known to contribute to symptomatic pain and a positive test for AC joint OA. Research demonstrated that heightened activation of this muscle correlates with increased joint stress, intensifying symptoms in patients with AC joint OA [
29,
30]. This understanding reinforces the importance of the deltoid clavicular muscle in diagnosing and managing AC joint OA.
However, during the CBA test, there were observable differences in the predictions of other muscle forces between OpenSim and AnyBody Modeling System
TM, such as the biceps short head, brachialis, coracobrachialis, pectoralis major, and triceps, as shown in
Figure 5. AnyBody Modeling System
TM and OpenSim might employ different assumptions regarding muscle activation patterns, recruitment strategies, and joint mechanics, which could lead to substantial differences in predicted forces. Further, research has pointed out that differences in anthropometric and anatomical definitions defined by each simulation tool can significantly affect muscle force predictions [
31]. These discrepancies are critical as they emphasize the necessity for precise and accurate modeling approaches to accurately accommodate the complexities of the human system and movement and predict the required kinetics [
32]. Moreover, the absence of serratus, sternocleidomastoid, and trapezius muscles in OpenSim’s model indicates a potential incompleteness of the model and questions its use in clinical tests like the CBA and HBB tests. Most significantly, the pectoralis major and trapezius have vector forces acting on the clavicle and across the AC joint based on their insertions. These vectors are crucial for shoulder adduction, elevation, and CBA motion, as the muscle forces stress the AC joint to delineate symptomatic pain while testing for AC osteoarthritis. The sternocleidomastoid, to a lesser extent, has a stabilizing effect on the clavicle’s medial end.
The HBB test results also highlighted agreements and discrepancies in muscle force predictions between the AnyBody Modeling System
TM and OpenSim, as shown in
Figure 6. The observable consistency in the brachialis and deltoid scapular muscle, along with similar activation patterns in the biceps long head, infraspinatus, pronator quadratus, pronator teres, supraspinatus, and triceps long head in each software, indicates their potential role during the HBB test. These repeated observations across both software assure researchers and clinicians that the deltoid muscle, a key contributor, is being accurately assessed by these models. Additionally, the accessory muscles that aid shoulder stabilization and arm positioning can be reliably predicted. However, the notable variation in force predictions for the supinator, teres minor, pectoralis minor, latissimus dorsi, pectoralis major clavicular head, biceps long head, infraspinatus, pronator teres, supraspinatus, and triceps long head underscores the potential differences in the algorithms used by the AnyBody Modeling System
TM and OpenSim to solve the muscle redundancy problem. The OpenSim model does not include the levator scapulae and trapezius clavicular muscle, the latter being a more significant omission due to its role in AC joint stabilization.
Another key aspect is the role of the accessory muscles during these movements. For instance, the pectoralis minor, serratus anterior, trapezius, and levator scapulae play a crucial role in stabilizing the shoulder girdle and supporting joint mechanics during diagnostic tests like the CBA and HBB tests. These muscles, while not directly crossing the AC joint, assist in maintaining scapulothoracic and clavicular stability, which is essential for reducing abnormal joint loading and ensuring proper force transmission through the shoulder complex. For example, the serratus anterior prevents scapular winging, and the trapezius aids in scapular retraction and elevation, both of which are vital for functional shoulder movements [
19]. Weakness in these muscles can exacerbate joint misalignment, increasing stress on the AC joint and worsening OA symptoms. Strengthening accessory muscles through targeted rehabilitation exercises, such as scapular stabilization and isometric holds, can improve shoulder stability, alleviate pain, and restore functional balance. This aligns with previous findings emphasizing the role of periarticular muscle strengthening in reducing joint degeneration and enhancing outcomes in OA patients [
33]. By incorporating accessory muscle-focused strategies into rehabilitation protocols, clinicians can optimize patient recovery and prevent further deterioration of the AC joint. Future studies using electromyography (EMG) can provide deeper insights into the contributions of these muscles to shoulder biomechanics and refine therapeutic approaches.
Additionally, differences in how each software models the path and geometry of muscles could have resulted in varied force predictions. Further, the variation in predicted forces for the same muscle across different software may reflect the complex interplay of muscle synergies-groups within the software to produce movement and influence the force-length-velocity relationship of muscles [
34,
35]. This hinders relying on one software to identify the potential muscles involved during the CBA and HBB tests. Additionally, even though this study does not consider age-related changes, there can also be significant differences in the muscle force prediction due to age factors. For instance, aging may result in reduced muscle strength, altered joint kinematics, and degenerative structural adaptations, further complicating AC joint OA diagnosis. To clinically correlate these muscle forces, a practitioner would also need to consider the morphology of the AC joint. This radiographic evaluation would involve decreased joint space, osteophytes, and surrounding bone sclerosis or remodeling that may elicit irritation to patients as the muscles evaluated in this simulation stress the joint’s degenerative nature in these clinical studies.
While these two tests may elicit symptomatic patients with AC joint OA, their discrepancies between muscle group activation may be a focus for rehabilitation in a clinical setting. Osteoarthritis is well studied in other joints, such as the hip and knee, and skeletal muscle wasting, proprioception, and muscle balance across joints have been factors noted to affect OA. Shorter et al. discuss that muscle weakness is not only an important determinant of pain and disability during OA, but it may also be linked with structural changes at the knee, leading to symptomatic pain in OA patients [
33]. They further discuss that therapeutic exercise to strengthen periarticular muscle may be beneficial in decreasing the regulation of certain biomarkers and improving cartilage health. Another study by Alkhamis et al. found that patients with unilateral hip OA had lower muscle strength, proprioceptive accuracy, and poorer functional balance than controls (
p < 0.003) [
36]. This indicates that a strong and balanced periarticular muscle group would be a rehabilitation treatment goal for patients with OA. For clinical treatment of patients with OA, whether in the hip, knee, or, in our case, the AC joint, rehabilitation protocols to strengthen the shoulder girdle muscles discussed and evaluated in our models could be a valuable option for patients to improve their pain and disability.
A significant limitation of this study is its theoretical nature and the lack of direct validation for the predicted muscle forces during the CBA and HBB tests against experimental data or the existing literature. While the models used in this study have been validated in previous research, they were not validated using subject-specific data for this particular investigation. This may limit the generalizability of the findings to individual patients in clinical settings, as variations in anatomy and biomechanics across different patients are crucial for personalized diagnostics and treatment planning. Although the simulations accurately represent general biomechanical movements, they may not fully capture the nuances of patient-specific conditions, potentially impacting their relevance for clinical decision-making and real-world biomechanics research. Additionally, the discrepancies observed between OpenSim and AnyBody Modeling System™ predictions highlight the limitations of relying solely on computational models for clinical decision-making. These discrepancies stem from differences in anatomical definitions, assumptions, and model implementations between the two platforms, leading to divergent predictions of muscle forces and activation patterns. So, there remains a gap in understanding how well these models reflect real-world muscle dynamics without validation through clinical trials or empirical data, such as motion capture or electromyography (EMG).
Future research should prioritize validating muscle force predictions and activation patterns through experimental methods, such as EMG and motion capture, to ensure alignment with actual physiological data. The first step in this process is to record the motion capture data for the CBA and HBB tests, which can then use these data as input in the musculoskeletal model to determine the kinematics and kinetics of the tests. The kinematics determines the angle and position of the joints, while the kinetics predicts the muscle forces required to maintain or perform the necessary motion. Simultaneously, EMG sensors need to be attached to the muscles of interest along with the motion capture system to measure their activation level during the CBA and HBB tests. Finally, we can compare the muscle forces/activation obtained from musculoskeletal modeling software and EMG output to identify the primary and accessory muscles involved during the CBA and HBB tests. This would not only validate the obtained results but also provide a more comprehensive understanding of shoulder biomechanics and muscle dynamics during the CBA and HBB tests. Musculoskeletal modeling also offers significant potential for enhancing both clinical and non-clinical aspects of orthopedic practice. Clinically, these models can improve diagnostic accuracy by quantifying muscle forces, activation patterns, and joint mechanics, enabling data-driven decisions, particularly in cases where subjective pain responses or imaging inconsistencies cause uncertainty. They also aid preoperative planning by simulating surgical outcomes, optimizing rehabilitation strategies, and tailoring interventions to individual patients. Non-clinically, musculoskeletal models serve as educational tools, helping clinicians and trainees visualize joint interactions and biomechanical principles while providing researchers with a platform to test hypotheses and refine diagnostic methods. Incorporating subject-specific data into these models would further improve their accuracy in clinical settings, where patient-specific anatomical variations are critical for effective diagnosis and treatment planning. Additionally, a pilot study to gather preliminary data on muscle forces using experimental methods would validate these predictions and serve as a foundation for integrating musculoskeletal modeling with finite element analysis (FEA). Using muscle force predictions as input for FEA can provide insights into joint mechanics, especially about the contact area and stress levels at the AC joint during the CBA and HBB tests, enhancing their clinical utility. Future research should then assess the reliability of the HBB test as a standalone diagnostic tool for AC joint OA. One approach could involve performing CBA and HBB tests on patients with AC joint OA and comparing the outcomes with radiographic studies to evaluate test sensitivity. Another idea could be comparing the stress level at the AC joint during CBA and HBB tests using the FEA mode. The test that results in a greater reduction in AC joint separation distance would have increased contact area at the joint and experience higher stress levels, indicating that it may be more effective for diagnosing AC joint OA. This will eventually contribute to advancing both clinical practice and orthopedic biomechanics of the AC joint.