1. Introduction
According to the available statistics, the economic losses caused by corrosion and friction-related phenomena, in industrialized countries, represent between 5 and 10% of the gross domestic product [1,2]. Hence, worldwide, several corrosion and wear mitigation strategies are taken into consideration to reduce the negative effects that are often associated with disasters, severe failure of equipment, environmental side effects, or increased maintenance costs [1]. One practical method for reducing superficial damage in engineering applications is to modify the surfaces of materials without changing their bulk properties [3]. Since various applications in the technical field require high resistance to aggressive conditions, different types of surface modifications, in the form of coatings, layers, or films, have been recently proposed to serve the increasingly exigent industrial expectations. Functional coatings are essential in a wide range of applications, starting from the food industry, medicine, aerospace, or agriculture [4]. The recent progress in coating technology, correlated with the economic impact generated by the mitigation of damaging effects, has led to a more intense interest of scientists in discovering novel coating systems with improved performances and innovative deposition techniques adapted to actual industrial needs [5,6,7]. Regardless of the class of materials they belong to, or the thicknesses they exhibit, generally, the coatings should provide important characteristics like resistance to corrosion, erosion, wear, adhesion to the substrate, mechanical strength, and/or oxidation resistance, without affecting the main function of the component. Furthermore, it has been demonstrated that some of the mentioned properties can be additionally improved by the incorporation of hard phases in metallic coatings [8]. In these circumstances, the coatings belong to the class of composite materials, consisting of a soft and ductile component as a matrix, and a harder, wear-resistant reinforcer usually in the form of particles or fibers. Composite coatings comprising refractory ceramic particles usually exhibit good wear properties [9]. Furthermore, the mechanical properties, as well as corrosion behavior, can be greatly improved in such composites compared to pure metallic coatings. Commonly, the strengthening effect in the metal matrix composites may be assigned to a set of long-range and short-range forces occurring from the presence of the second-phase particles [10]. The particle interaction in the composite coating was found to be important in dislocation due to the different elastic properties of the two constituents and the local resistance of the particles as obstacles to the dislocation motion. The improved properties mainly depend on the ceramic particle content and the nature of the metal matrix. Moreover, by embedding ceramic particles into a metallic matrix, properties such as self-lubrication [11] and corrosion resistance [12] have also been significantly improved [13].
Recent advancements on this topic include the development of nanocomposite coatings, which offer even greater performance by incorporating nanoscale reinforcements [14]. Additionally, functionally graded materials are being explored to create coatings with varying properties across their thickness, optimizing them for specific applications. Although it is possible to obtain composite coatings by various deposition techniques [15,16,17,18,19,20], like laser cladding [21], thermal spraying [22], laser melt injection [23], brazing [24] and electrospark coating deposition [25], the electrodeposition process is preferred in some cases due to the several advantages provided, such as ease of application, reproducibility, low cost, and high efficiency compared to other coating technologies [26]. Particularly, the process applied for depositing charged ceramic particles from an aqueous-based colloidal electrolyte onto a conductive substrate is called electrophoretic deposition (EPD). It is usually employed to deposit high-purity ceramic coatings for biomedical applications and is commonly followed by a sintering process [27]. Recently, this deposition process was adapted to co-deposit the ceramic particles along with metals and alloys, in order to form composite coatings. In this case, the deposition can be considered a hybrid one, combining the advantages of the electrolytic and the electrophoretic deposition in one step. The main parameters of the suspension that may influence the morphology of the resulting coating include particle size, electrical conductivity, viscosity, or stability of the suspension. Deposition parameters, like duration, applied voltage, deposition type and conductivity of the substrate, also have significant effects on the process.
Among the metals that benefit from this deposition technology is cobalt (Co) [28], which is known for its remarkable properties, like hardness and high melting point, excellent resistance to corrosion and wear, as well as exceptional mechanical, electrical, magnetic, and chemical properties [8]. In this context, Co is recommended for replacing hard chromium coatings, for the development of coatings with practical applicability in gas turbines, automotive, medical, chemical, and petroleum industries, as well as chemical catalysis [29]. Considerable efforts have also been made to adopt environmentally friendly processes, to address the challenges related to the high cost, health, and safety concerns associated with cobalt-based coatings [30,31]. Additionally, Co-based composite coatings are relatively less expensive compared to other composite coatings such as nickel-based composites, epoxy/alumina composites, or copper-based composites [8]. Nowadays, Co-based composite coatings are increasingly vital for enhancing the durability and performance of industrial components subjected to extreme conditions. These coatings, composed of a Co matrix with embedded reinforcing particles like carbides [32], oxides [33], polymers [34], or graphene/carbon fibers [35], are known for their exceptional wear behavior, corrosion resistance, and stability at high temperatures. A series of studies have explored the fabrication and properties of Co-based composite coatings deposited by laser cladding technique [36,37,38]. Weng [36] and Yan [37] both found that the addition of CeO2 and CaF2 particles, respectively, improved the microhardness and wear resistance of the Co-based coatings. Weng et al. [38] further enhanced these properties by adding Ti5Si3 and TiC. Toosinezhad et al. [39] developed composite layers based on pure cobalt and cobalt/graphene produced by electrodeposition technique and found out that both types of coatings presented a higher hardness compared to the substrate material, while the cobalt/graphene microhardness was around 3 times higher than the pure Co coating. Ramesh Bapu and Thiruchelvam [40] found that Co-BN composites exhibited improved corrosion resistance in NaCl and HCl solutions compared to pure cobalt. They established that factors affecting particle incorporation in these composites include bath chemical composition, current density, pH, and temperature. Toosinezhad et al. [41] developed Co-graphene composite coatings deposited on a St37 steel substrate which exhibited increased hardness, better tribological behavior, and improved corrosion resistance in NaCl solution compared with the base material.
Based on the proven potential of Co composite coatings, the current work aims to evaluate the possibility of obtaining Co-diamond composites in a single-step hybrid electrophoretic deposition. The effect of the diamond particle incorporation into the electrochemically deposited cobalt coatings is evaluated, analyzing the most significant characteristics like sliding wear and corrosion behavior. The results were compared with a pure cobalt coating obtained in similar deposition conditions, as well as with the low-alloy steel used as substrate.
2. Materials and Methods
The substrate material employed as support for the cathodic electrodeposition of the Co-based coatings was a commercial S235 structural carbon steel cut into rectangular coupons of 20 mm × 20 mm × 5 mm. The chemical composition of the S235 steel was established using an optical emission spectrometer (OES) (Thermo Electron Corporation Genesys, Thermo Fisher Scientific, Waltham, MA, USA), and the values are presented in Table 1. Before deposition, the surface of the steel substrate was mechanically polished using various grades of abrasive paper to achieve a mirror-like metallic surface with a roughness of Ra = 0.2 µm, followed by ultrasonic cleaning in distilled water and ethanol. Subsequently, after drying, the surface was soaked in 2M HCl solution at 25 °C for 15 s, to remove the residual oxides, followed by an additional rinse with distilled water.
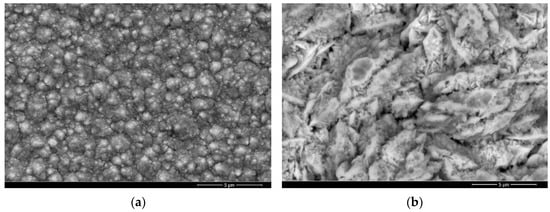
To improve the adhesion of the Co coating to the steel substrate, an intermediate thin nickel layer (5 μm) was first electrochemically deposited onto the steel surface, from a typical Watts solution. The electrochemical deposition of both Ni and Co was performed in a two-electrode cell configuration, with continuous stirring, with the aid of a programmable direct current power supply (Voltcraft PPS-16005 36 V, 10 A, Conrad Electronic SE, Hirschau, Germany). The deposition electrolytes were prepared by dissolving the commercially available chemical reagents in distilled water. The composition of the Ni and Co electrolyte, as well as the working conditions, are presented in Table 2. Additionally, to produce the Co composite coatings, diamond particles (0.5 μm) were added to the Co-based electrolyte, generating a 2.5 g L−1 concentrated suspension. The diamond particles are therefore co-deposited in the Co coating during the electrochemical deposition process. The deposition rate and coating thickness were controlled by varying the applied current and the deposition time. While the role of the Ni and Co salts in the deposition electrolyte is as a source of metal ions, the H3BO3 is added to stabilize the pH during the deposition, near the electrode surface. The working conditions were experimentally adjusted to obtain a uniform Co-based coating, with a thickness of around 50 μm. The morphology and microstructure of the electroplated samples were analyzed using an FEI Quanta™ FEG 250 (FEI Quanta™, Hillsboro, OR, USA) scanning electron microscope (SEM) equipped with energy-dispersive spectroscopy analyzer (EDS) for chemical composition identification. The roughness of the surface before the electrochemical deposition was evaluated with a Mitutoyo SJ-201 portable surface tester (Mitutoyo, Aurora, IL, USA). The thickness of the coatings was checked in cross-section, using SEM analysis.
The corrosion resistance of the Co-based coatings with and without diamond particles was investigated by potentiodynamic polarization, in a 3.5 wt.% NaCl solution, and compared to the S235 steel substrate, as reference. All electrochemical measurements were carried out in a three-electrode corrosion cell connected to a potentiostat/galvanostat (Biologic SP 150, Biologic, France). The working electrode consisted of the Co-based coated samples (1 cm2 exposed surface), the reference was a Ag/AgCl (3M NaCl) electrode positioned near the working electrode via a Luggin capillary, and the counter electrode was a Pt gauze. The potentiodynamic polarization curves were recorded at normal temperature with a scan rate of 0.16 mV s−1 by sweeping the potential between ± 500 mV and the open circuit potential (OCP). The main corrosion parameters were evaluated from the obtained polarization curves using the Tafel extrapolation method in the linear region of the anodic and cathodic branches and compared to the steel substrate. The microhardness of the coatings and substrate was evaluated on the polished cross-sectioned sample using a Vickers indenter (ZHVμ device from Zwick/Roell, Ulm, Germany). The reported values (HV0.3) represent the average of five measurements, each performed under a vertical load of 30 g for a duration of 15 s. The sliding wear behavior of the steel substrate and the Co-based coatings was evaluated using the ball-on-disc method, according to the ASTM standard G99 [42]. The investigations were performed with the aid of a TR-20 Tribometer, (Ducom-Materials Characterisation Systems), under dry sliding conditions and ambient temperature, setting a normal load of 5 N, sliding rate of 100 rot min−1, wear track diameter D = 10 mm, test time of 100 min, and a total sliding distance 314 m. The counterbody selected for the tests was a 100Cr6 steel ball with a 6 mm diameter. During the tests, the coefficient of friction (COF) variation with the distance was automatically recorded. The wear track profile after the tests was subsequently analyzed, and the wear rates were estimated. For each type of sample, three measurements were performed in order to ensure the reproducibility of the results.
Source link
Diana Uțu www.mdpi.com