1. Introduction
The solid oxide fuel cell (SOFC) is an electrochemical device that directly converts chemical energy in fuel into electrical energy through an electrode reaction and is a very promising new energy technology for efficient power generation based on existing energy supply systems [1]. Considering the high operating temperature of traditional SOFCs, cell encapsulation is hard to perform, and the production cost is high. Therefore, the development of IT-SOFCs that can operate at medium temperature is one of the current research hotspots [2]. However, as the operating temperature decreases, the polarization impedance of the cathode increases significantly, thus mainly restricting the electrochemical performance of IT-SOFCs [3]. Therefore, researchers have focused on improving the output performance of SOFCs under medium-temperature conditions by studying excellent cathodes. Currently, for anode-supported SOFCs, the slow oxygen reduction reaction (ORR) at the cathode dominates the polarization loss of the cell [4,5]. The lack of efficient, low-cost, and stable cathode materials is an urgent problem that needs to be solved. In recent years, the LnBaCoO5+δ-type double perovskite oxide, which contains the rare earth element Ln, has been widely studied as an SOFC cathode material because of its special layered structure, resulting in its excellent conductivity and oxygen transport properties [6]. However, Co-based double perovskites generally encounter problems such as a high average thermal expansion coefficient (TEC), poor stability, and high cost [7,8]. The Fe-based perovskite oxide is among the most potential alternative materials for cobalt-based cathodes because of its high reserves in nature, low cost, and good resistance to CO2 and vapor [9]. However, it suffers from poor electrocatalytic activity, thus requiring the optimization of more materials as IT-SOFC cathode materials.
Considering the existence of various valences of B-site transition metal ions, the valence of ions can be adjusted intuitively by B-site doping to change the crystal structure, affect the oxygen ion diffusion, and improve the catalytic activity. In addition, the B-site doping of the perovskite structure oxide with stable ions can improve the stability of the cathode material in a high-temperature environment and cathode atmosphere (CO2 and humid air) and effectively reduce the TEC of the cathode material. The most common doping materials are Cu, Fe, Ni, and Mn [10,11]. The incorporation of high-valence cations such as Nb5+, Ta5+, and W6+ can effectively stabilize the phase structure of peroxides, reduce the TEC, improve the thermal matching with other devices, enhance the catalytic activity, increase the output power, and raise the life of cells [12,13,14]. Some Fe- and Co-based peroxides are often doped with Mo to improve the electrochemical performance [15,16]. Therefore, in this paper, LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1) was prepared by doping high-valence Mo at the B position, and the effects of Mo doping on the phase structure, thermal expansion characteristics, conductivity, and electrochemical properties were studied in detail.
2. Results and Discussion
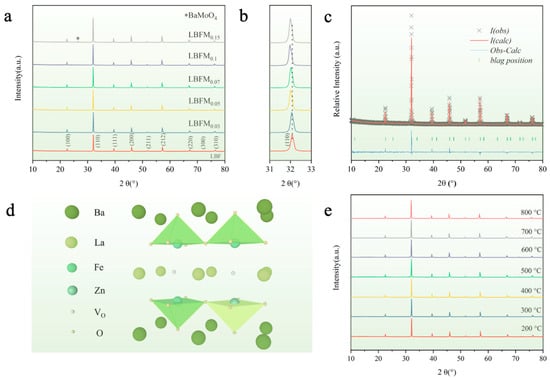
Figure 1a shows the XRD patterns of the sample LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1, 0.15) after calcination at 1100 °C. No impurity phase was obtained when the doping amount is less than 0.1, indicating that LBFMx is in a pure double perovskite phase [17]. The XRD diffraction peaks of LBFMx gradually shifted to a small angle with the increase of Mo doping as shown in Figure 1b, which indicates that the cell volume of LBFMx gradually increases and the lattice expands with the increase of doping. The impurity phase BaMoO4 (PDF.#8-455) was detected as the doping amount was increased to over 0.1 [18]. The crystal structure of LBFMx was determined by refining the XRD patterns by using the GSAS/EXPGUI software (PC-GSAS) (Figure 1c and Figure S1), which showed a good match with the XRD pattern, and the refinement results are listed in Table S1. The results show that the cell parameter of LBFMx gradually increased with the increase in doping amount, and the cell volume increased, because the Mo6+ radius (0.59 Å) and Fe4+ radius (0.585 radius) are close [19]. The Fe3+ content increased after the B-site Fe was replaced by Mo, thus increasing the cell volume. The results show that LBFMx samples have the same structure as the undoped LBF, indicating that Mo doping does not change the original crystal structure. Figure 1d shows the crystal structure of LBFM0.1. The LnO and BaO layers are arranged in alternating layers of -BaO-FeMoO2-LaOδ-FeMoO2-BaO- along the C-axis, and oxygen vacancies mainly form and migrate in the LaOδ layer. The HT-XRD patterns of the material under an air atmosphere in the range of 200 to 800 °C were tested to assess the thermal stability of the materials, and Figure 1e shows that the materials are all in a single phase, indicating that the materials have a good thermal stability.
Figure 2a shows the energy-dispersive spectroscopy (EDS) diagram of LBFM0.1 cathode powder attached to the CGO electrolyte, showing that La, Ba, Fe, Mo, and O are evenly distributed, and no element aggregation is found. This finding confirms that Mo successfully replaces Fe at the B-site. LBFMx has a tetragonal encapsulated crystal structure in the P4/mmm space group, and sample LBFM0.1 was characterized using transmission electron microscope as shown in Figure 2b, where the lattice spacings of 0.39 nm and 0.78 nm correspond to the (010) and (001) crystallographic planes, respectively.
During the operation of SOFCs, oxygen ions are transferred from the cathode layer to the electrolyte layer; consequently, the poor chemical compatibility between the cathode layer and the electrolyte may create an insulating layer at the cathode/electrolyte interface, thus greatly reducing the performance of SOFCs [20]. The LBFM0.1 sample and CGO powder were mixed at a mass ratio of 1:1 and sintered at 1100 °C for 5 h. The sintered CGO-LBFM0.1 mixture was studied by XRD, and the results are shown in Figure S2. Compared with the XRD data of the single-phase LBFM0.1 and CGO, the diffraction peak of the mixture after sintering has no obvious shift, and no new phase formed after high-temperature heating, indicating that it has a good chemical compatibility with the electrolyte CGO at high temperature.
The TGA curves of the LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1) series cathode powders from 30 °C to 800 °C are shown in Figure 3a. The decrease in mass between 30 °C and 300 °C is attributed to the loss of water and gas at the surface of the cathode material [21]. At temperatures higher than 300 °C, this phenomenon is mainly caused by the escape in lattice oxygen accompanied by the generation of oxygen vacancies [22], resulting in a decrease in mass, as shown in Equation (1).
, , , and represent Fe4+, Fe3+, and the lattice oxygen and oxygen vacancy. As shown in Figure 3a, the increase in doping amount will increase the weight loss because of the water absorbed by the material and oxygen adsorbed on the surface, and the increase in surface oxygen vacancy will decrease the weight. According to the TGA curve from 30 °C to 800 °C, the weight loss rate of a series of cathode materials is only between 1.3% and 2.0% during heating, confirming that the prepared materials have a high structural stability. The iodimetric titration results show that the oxygen non-stoichiometric ratio of the sample decreased with the increase in doping amount (Table S2). The change curve of the oxygen content and temperature of LBFMx was obtained by combining the TGA data, as shown in Figure 3b. The oxygen content gradually decreased with the increase in doping amount, indicating that the oxygen vacancy of the material gradually increased after substitution. Initially, the oxygen content of the sample remains unchanged with the increase in temperature, possibly because oxygen ions are frozen in the lattice at low temperature [23]. With the further increase in temperature, the oxygen content of the sample gradually decreases, and this phenomenon is related to the release of lattice oxygen caused by high temperature.
The chemical valence states of surface ions of LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1) series materials were analyzed by XPS, because the cationic oxidation state is an important factor that affects the catalytic activity of the cathode. As shown in Figure 4a, the high-resolution map of O1s can fit four different characteristic peaks, indicating the existence of different types of oxygen species [24]. The diffraction peak at 528.35 eV (±0.2 eV) can be attributed to lattice oxygen (Olat). The peak at 529.3–531.12 eV (±0.2 eV) is related to the adsorbed oxygen (O2−O− and OH−/CO32−), while the peak at 532.11 eV (± 0.2 eV) belongs to Ovacancy and moisture oxygen (OH). The ratio between the adsorbed oxygen (OC) and lattice oxygen (Olat) reflects the content of surface oxygen vacancies. Therefore, the calculated OC/Olat ratio is listed in Table S3, and its ratio is relatively increased with the increase in doping amount, indicating that Mo can replace Fe at the B-site to obtain more oxygen vacancies and oxygen adsorption/dissociation active centers. In the LBF lattice, the holes generated by Fe3+/Fe4+ are electron carriers and provide Fe3+–O2−– Fe4+ electron transport paths for hole transport [25]. Therefore, the valence state of Fe in LBFMx materials needs to be studied. Figure 4b shows the high-resolution XPS map and fitting curve of Fe2p at room temperature. The binding energy is decomposed into two different peaks at 710.2 eV (±0.15 eV) and 711.2 eV (±0.26 eV), corresponding to Fe3+2p3/2 and Fe4+2p3/2, respectively. The binding energies of 722.9 eV (±0.14 eV) and 724.2 eV (±0.25 eV) correspond to Fe3+2p1/2 and Fe4+2p1/2, respectively [26,27]. The percentage values of Fe3+ and Fe4+ are listed in Table S3. With the increase in the Mo doping amount, the content of Fe4+ decreased, and the average valence state of Fe gradually decreased.
For the understanding of the effect of Mo doping on oxygen ion and hole conduction, the conductivity of LBFMx (x = 0 and 0.01) was measured from 200 °C to 800 °C in air, and the results are shown in Figure 5. At 400 °C, both samples showed an increasing trend of electrical conductivity with increasing temperature, which follows the small polaron conduction mechanism [28]. The electrical conductivity decreased with increasing temperature at 400–800 °C, because the oxygen content decreased with increasing temperature, and a large amount of lattice oxygen is released. Subsequently, the carrier concentration gradually decreased. At 400 °C, the conductivities of LBF and LBFM0.1 are 49 and 42 s·cm−1, respectively. The conductivity decreased with the increase in Mo doping concentration. This finding can be explained by the following reasons: According to the principle of charge conservation, Mo doping at the B-site causes a partial reduction of Fe4+ to Fe3+, which is confirmed by the XPS results, and this phenomenon leads to a decrease in the concentration of electron holes. Therefore, the conductivity of electrons decreased. Second, the electron conduction in peroxides follows the small-pole jump conduction mechanism and is conducted by the Bn+1-O2−-Bn−1 net structure, and the non-conductive Mo-O bond increases with the increase in Mo substitution, thus hindering the electron transport and decreasing the conductivity [19,29].
Figure 6 shows the thermal expansion curves of LBFMx at 30–750 °C in air. Based on the figure, the relationship curve between the thermal expansion degree (dL/L0) and temperature is not completely linear. The mechanism of the thermal expansion behavior of oxides is different at high and low temperature, and the thermal expansion at low temperature is related to physical expansion. However, the thermal expansion at the high-temperature range is influenced by both physical and chemical expansion. In addition, the chemical expansion is related to the reduction of Fe in the B-site and the formation of oxygen vacancies in the sample [18,30]. According to the figure, the thermal expansion of the sample increases with the increase in temperature, and the increase in TEC corresponds to the crystal expansion caused by harmonic atomic vibration. When the temperature is above 300 °C, the rapid increase in TEC is caused by the Fe decrease and the formation of oxygen vacancies in the sample. This process can be represented by Equation (1). As shown in Table S4, the average TEC of the sample gradually decreased with increasing Mo doping concentration, indicating that Mo doping increased the thermal stability. This increase in thermal stability can be attributed to the stronger binding strength of Mo–O (359.92 kJ·mol−1) than that of Fe–O (200.15 kJ·mol−1) [18]. Furthermore, the incorporation of Mo reduced the amount of Fe4+ in the sample, which also favored the TEC reduction. The average TEC values for LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1) at 30–750 °C are 13.2 × 10−6·K−1, 12.1 × 10−6·K−1, 11.8 × 10−6·K−1, 9.73 × 10−6·K−1, and 9.09 × 10−6·K−1. These values are compatible with the widely used CGO electrolyte materials (TEC = 11.9 × 10−6 K−1) and are substantially lower than those of cobalt-based peroxide materials, such as BaBi0.05Co0.8Nb0.15O3-δ (19.6 × 10−6 K−1, 30–800 °C) and BaCo0.7Fe0.2Nb0.1O3-δ (24.3 × 10−6 K−1, 50–800 °C) [31,32]. The incorporation of Mo in LaBaFe2O5+δ significantly decreased the TEC, especially at the high–temperature range. The average TEC between 300 °C and 750 °C decreases from 21.2 × 10−6 K−1 to 14.5 × 10−6 K−1, corresponding to a change of 26%. It could improve the thermal compatibility between the cathode and electrolyte and improve the long-term cyclic stability of SOFCs.
Figure 7 and Figure S3 show the SEM images of symmetric cell sections LBFMx|CGO (x = 0 and 0.1). The samples are all loose and porous, which is conducive to gas diffusion, oxygen ion transport, and charge transfer. In addition, the cathode material is well-attached to the electrolyte CGO, and no obvious cracking and delamination was observed between the electrolyte and the cathode material, confirming that it has a good chemical compatibility with the electrolyte.
The activity and effectiveness of LBFM0.1 as an SOFC oxygen electrode were evaluated by preparing a symmetric cell with CGO as the electrolyte, and the sample was named LBFM0.1|CGO|LBFM0.1. The electrochemical impedance spectroscopy (EIS) curve measured in the range of 600–800 °C under the air condition is shown in Figure 8a. The equivalent circuit diagram Rs (RHF//CPEHF RMF//CPEMF RLF//CPELF) was used to fit the measured AC impedance profile. Rs represents the Ohmic resistance. RHF, RMF, RLF, and Rp correspond to high-, intermediate-, and low-frequency polarization resistance and total resistance Rp (Rp = RHF + RMF + RLF), respectively [33,34]. The effect of Mo doping on the electrochemical properties is obtained by preparing a symmetric cell LBFMx|CGO|LBFMx (x = 0, 0.03, 0.05, 0.07, 0.1). The AC impedance spectra in the range of 600–800 °C were obtained. The above equivalent circuit diagram was used for fitting, and the results are shown in Table S5. Figure 8b shows the AC impedance spectrum at 800 °C. The Rp value gradually decreased with the increase in doping amount, because Mo doping increases the oxygen vacancy concentration of the sample, promotes oxygen adsorption and deionization, improves surface oxygen exchange and oxygen ion diffusion, and is conducive to the ORR process [35,36]. Figure 8c shows the Arrhenius diagram of Rp values of LBFMx at 600–800 °C, with Ea values of 195.6, 173.7, 151.7, 141.2, and 136 kJ·mol−1. This finding indicates that Mo substitution for Fe can promote the oxygen reduction reaction. The electrochemical reduction reaction of oxygen on the cathode surface and at the cathode/electrolyte interface are complex processes [37,38]. The specific role of Mo doping at the B-site was determined by calculating the Rp values of LBFMx|CGO|LBFMx at 800 °C in the high-, middle-, and low-frequency bands as shown in Figure 8d. Compared with LBF, the Rp values of LBFM0.1 corresponding to the high-, middle-, and low-frequency region significantly decreased and is in the order of RHF > RLF > RMF, indicating that the transfer of oxygen ions is the rate control step of the ORR process.
The effect of doping Mo on the output performance of a single cell was compared by preparing the anodic supported single cell with CGO as the electrolyte, and the structure is shown in Figure 9a. By using humidified hydrogen as the fuel gas and having the cathode side in direct contact with air, a single-cell LBFMx|CGO|NiO + CGO (x = 0 and 0.1) was tested. The I–V–P curves at 650–800 °C are shown in Figure 9b,c. Figure 9d shows the measured peak power density (PPD) of a single cell at different temperatures. The PPD value of LBFM0.1|CGO|NiO + CGO is 599 mW·cm−2 at 800 °C, which is about 46% higher than that of LBF at the same temperature, possibly because of the low polarization resistance of LBFM0.1, indicating that the Mo substitution of Fe at the B-site improves the electrochemical performance [19]. Figure S4 shows the SEM image of the single-cell LBFM0.1|CGO|NiO + CGO after the test. No obvious delamination was observed between the components, indicating the good chemical compatibility and thermal matching between components. The electrolyte layer after sintering at high temperature is relatively dense, ensuring a high open-circuit voltage.
Source link
Liangmei Xue www.mdpi.com