1. Introduction
With the rapid development of modern metallurgy, battery, and energy industries, some heavy metals, such as lead and mercury, have entered the water bodies, causing serious environmental pollution. In order to cope with this challenge, countries all over the world have taken measures to strengthen the investment of water restoration work in order to improve the control capacity of heavy metal pollution.
At present, the treatment methods for heavy metal ions such as lead and mercury in industrial sewage include the adsorption method, the electrochemical method, the microbial degradation method, the membrane filtration method, and others [
1,
2]. Among them, the adsorption method has been widely used, boasting advantages in terms of low energy consumption, convenient operation [
3,
4], and renewable and low-cost materials. Commonly used adsorbents include natural porous materials, metal–organic frameworks, covalent organic frameworks, activated carbon, and nanoparticle materials [
5,
6,
7,
8]. Generally speaking, high-performance adsorbents usually have the characteristics of large specific surface area, good pore structure matching, good stability, and easy production, but in industrial wastewater treatment, the repeated recycling of adsorbent is an important index by which to evaluate its practicability [
9]. The traditional filtration and centrifugal method is complicated, costly, and less effective. Therefore, there is an urgent need to develop a viable adsorbent that can be separated from the environment and easily collected.
In recent years, the synthesis and application of magnetic nanoparticles (MNPs) with outstanding advantages, such as supermagnetic performance, low toxicity, high specific surface area, and being easy to recover, have attracted widespread attention [
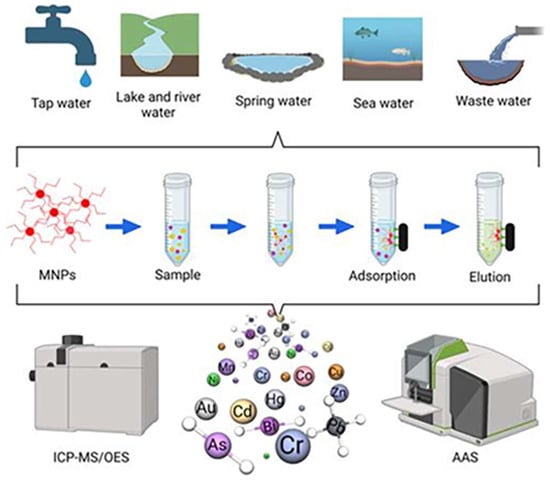
10]. Researchers, by modifying the surface of MNPs, not only improve their adsorption capacity, renewable ability, target selectivity, and stability in acidic medium; they also avoid the aggregation of magnetic particles in the treatment of heavy metal ions and pollutants in water samples (the application of the magnetic nanoadsorbent and the detection method are shown in
Figure 1). The modified materials include sponges, hydrogels, carbon nanotubes, graphene/graphene oxide, molecularly imprinted polymers, covalent organic frameworks, layered double hydroxides, metal–organic frameworks, organic compounds containing functional groups, etc. The formed magnetic nanoadsorbents are represented by sponges, beads, CNTs, G/GO, MIPs, COFs, LDHs, MOFs, and FUN, respectively, and their structures are shown in
Figure 2.
The magnetic sponge (Spongs) is mainly used for oil pollution removal and oil–water separation, and the adsorbent is poorly functionalized in the preparation process. The application is limited to some extent [
11]. The magnetic beads (Beads) are mainly used for softening water. Preprocessing is required before its use, and the procedure is highly complicated [
12]. Magnetic carbon nanotubes (CNTs) and graphene/graphene oxide (G/GO) are ideal for extracting and concentrating organic pollutants from various matrices, but there is a high preparation cost for this type of adsorbent, as well as poor dispersity [
13,
14]. Magnetic molecular imprinted polymers (MIPs) can act as selective adsorbents for some compounds and ions and are well suited for the selective extraction of fluorine/chlorine compounds, herbicides, and flavonoids. However, the disadvantage is that it is difficult to prepare, and the functional groups are difficult to introduce [
6,
15]. Magnetic covalent organic frameworks (COFs) have the advantages of selective and tunable porosity, easy functionalization, satisfactory chemical and thermal stability, a large specific surface area, and ordered channels. However, the disadvantages are limitations in the rapid, intuitive, and quantitative analysis of harmful substances [
7,
8,
16]. Layard double hydroxides (LDHs) are suitable for water treatment and the purification industry, but the disadvantages are obvious; one is that the number of active sites is small; another is that to enhance activity, we can only increase the lateral size and thickness, resulting in excess [
17,
18]. Magnetic metal–organic frameworks (MOFs) have a large surface area and different porosities; multiple functional groups in structure; high thermal stability; and adjustable shape, size, and selectivity. They are widely used to separate toxic substances in gases, liquids, and environmental samples and to remove metal ions and polar and nonpolar organic matter. But such adsorbents are usually limited by pore size and are unstable [
19,
20,
21].
Magnetic nanoadsorbents with grafting functional groups (FUN) are usually coated with silica gel on the surface of MNPs; then, functional groups are grafted onto the silica gel. Silicone is not only an adsorbent material that can adsorb more heavy metal ions; it also has a tight and stable binding with MNPs. At the same time, silicone reduces the aggregation of MNPs. Functional groups are generally efficient heavy metal ion coordination groups, which have good adsorption capacity for heavy metal ions. In addition, FUN have the advantages of a large specific surface area, strong adsorption capacity, uniform dispersion, stable structure, renewability, and convenient recovery, and they are widely used for adsorbing heavy metal ions in wastewater [
22,
23,
24].
Generally, how high-performance functional groups such as pyridyl and thiol groups can be quickly and efficiently grafted onto adsorbents is a key consideration for researchers when preparing FUN. In this article, a novel FUN adsorbent, Fe
3O
4@SiO
2-yl-VP, was rapidly and effectively synthesized through an addition reaction between Fe
3O
4 nanoparticles coated with allyl silica gel and 4-pyridine ethylene. Usually, functional groups are grafted onto the surface of magnetic materials through substitution reactions or simple mixing under adhesive conditions [
25,
26,
27,
28,
29]. We attempted an addition reaction and successfully synthesized an adsorbent, providing a new method for the synthesis of FUN. Fe
3O
4@SiO
2-yl-VP not only has a low preparation cost and a simple and fast synthesis method but has also shown excellent adsorption performance, renewable performance, and convenient recovery in extracting Hg (II) and Pb (II) from industrial wastewater, making it a promising heavy metal ion adsorbent.
4. Conclusions
Compared to the usual preparation of magnetic nanoadsorbents through substitution reactions and simple mixing under bonding conditions, we have successfully prepared Fe3O4@SiO2-yl-VP using a novel addition reaction.
Fe3O4@SiO2-yl-VP not only has low preparation cost and a simple and fast synthesis method; it has paramagnetism. After the experiment is completed, it is easy for Fe3O4@SiO2-yl-VP to magnetically separate from the solution, achieving maximum adsorption capacities of 85.06 mg/g and 73.78 mg/g for Hg (II) and Pb (II) in just half an hour at pH = 5 and pH = 7, respectively. The adsorption process conforms to the Langmuir model, pseudo-first-order kinetic model, and the pseudo-second-order kinetic model.
Magnetic clusters with good homogeneity are obtained (if this aspect is to be proven by subsequent TEM measurements). Magnetic clusters show superparamagnetic properties, with saturation magnetization values suitable for magnetic separation applications. And finally, with respect to the adsorptions of the two metals, with reference to the literature, similar, lower, and higher values were obtained.