1. Introduction
Hemovigilance significantly reduces the risk of transfusion-transmitted infections (TTIs) [
1]. Despite this progress, only 66% of countries have reported having specific legislation to ensure the safety and quality of blood transfusions [
2]. Globally, approximately 118.5 million blood transfusions are performed annually worldwide [
3,
4]. According to the World Health Organization (WHO) recommendations, it is crucial to detect HIV, hepatitis B (HBV), hepatitis C (HCV), and syphilis in potential donors to ensure the safety of the blood supply and prevent the transmission of these infectious diseases through transfusion [
3,
4].
Blood transfusion is a common procedure in inpatient settings, particularly in tertiary care hospitals, where transfusion therapy is essential due to specialized surgical services. The data indicate that 26.3% of critically ill patients received one or more transfusions during their ICU stay, with a median of 2 units per patient [
5]. Despite these benefits, transfusions carry risks, necessitating rigorous testing to ensure their safety and quality. These tests include serological tests using chemiluminescence and nucleic acid testing (NAT/TMA) in individual samples, with confirmatory tests such as Western blotting for HIV and recombinant immunoblot assays for HCV [
6].
Given the global context of TTIs and the critical need for stringent testing, it is important to examine the prevalence of these infections worldwide. Globally, there are 39 million cases of HIV [
7], 8 million cases of syphilis [
8], 254 million cases of hepatitis B, and 50 million cases of hepatitis C [
9]. Despite overall control in recent decades, the WHO reported an increase in HIV and syphilis in 2022, particularly in Latin America [
7]. This is concerning, as a significant percentage of individuals, such as 15% of HIV patients, remain undiagnosed, contributing to ongoing transmission [
7].
In Mexico, the prevalence rates in adults in general population were 0.17% for HIV, 1.4% for syphilis, 0.15% for hepatitis B, and 0.48% for hepatitis C [
10,
11]. National estimates suggest that there are over 17,858 cases of HIV, 411,000 cases of hepatitis B, 46,000 cases of active hepatitis C, and 7015 cases of syphilis.
Advanced screening tests such as NAT enhance transfusion safety by reducing the infectious window period for HIV-1, HCV, and HBV, allowing for earlier detection and improved overall safety. Incorporating NATs into blood screening protocols is crucial for mitigating transfusion risks and ensuring patient safety [
6,
11,
12].
At the National Institute of Cardiology Ignacio Chávez (INCICH), various types of blood donors contribute to the blood supply, emphasizing the need for rigorous screening. At the INCICH, there are three types of blood donors: voluntary and altruistic, replacement, and remunerated. Voluntary donations are the safest and most commonly encouraged. The donation rate is 6.6 per 1000 people in middle-income countries and 5.0 per 1000 people in low-income countries, with up to 50% of donated blood coming from family members and close associates [
11,
13,
14]. In Mexico, it is estimated that 1% of the population comprises blood donors, translating to approximately 1.3 million donations per year [
11].
The importance of an efficient blood bank at INCICH is evident given its role in complex cardiovascular care. INCICH, a major tertiary care hospital in Mexico, relies on an efficient blood bank to support complex cardiovascular care, including cardiac surgery. Over a 10-year period, from 2012 to 2022, the blood bank received more than 100,000 donors. Donors are required to meet specific criteria, such as not having suffered from hepatitis, syphilis, or HIV, and avoiding risky practices, such as tattoos or recent surgeries.
Continuous monitoring at INCICH provides valuable data on trends. Monitoring the prevalence and trends of TTIs among blood donors at INCICH from 2012 to 2022 provides valuable epidemiological surveillance data for the institution. This ongoing monitoring helps to understand and respond to the changing landscape of infectious diseases among blood donors at INCICH.
Building on previous studies, this study aims to provide a comprehensive and updated analysis. While previous studies have examined TTIs in Mexican blood donors, this study aimed to provide a comprehensive and updated analysis at INCICH from 2012 to 2022. We evaluated the prevalence of HIV, syphilis, hepatitis B, and hepatitis C among potential blood donors at this major tertiary care center.
2. Materials and Methods
2.1. Study Design
A total of 117,756 blood samples from “apparently healthy” potential donors were analyzed at the Blood Bank of the National Institute of Cardiology Ignacio Chávez (INCICH) between 2012 and 2022. This cross-sectional study assessed the prevalence of transfusion-transmissible infections in the donor population and compared the prevalence according to gender.
2.2. Participants
Before blood sampling, potential donors completed a questionnaire that included sociodemographic data, geographic indicators, personal and medical history, gynecological and obstetric history, risk factors and practices (such as tattoos, acupuncture, piercings, and previous sexually transmitted infections), and current health status. All the donors signed an informed consent form for donation. The inclusion criteria for blood donation at INCICH were being from 18 to 65 years old, weighing at least 50 kg, in general good health, presenting official photo identification, and fasting for a minimum of 4 h. Donors were excluded if they had epilepsy, hepatitis, syphilis, malaria, cancer, AIDS, or severe heart disease. Additionally, those who had consumed alcoholic beverages in the past 48 h; had undergone any type of surgery in the past six months; had tattoos, piercings, or acupuncture in the past year; or had been vaccinated against hepatitis or rabies in the past year were excluded.
2.3. Sample Size and Sampling
We included all information of potential blood donors who attended during the period 2012–2022. To ensure adequate precision in estimating the prevalence of infections, we performed precision estimation via EPIDATA software Version 4.2. Our analysis indicated that using data from more than 100,000 participants allowed us to achieve an excellent precision measure of less than 0.01.
This study was approved by the Research Committee of INCICH, ensuring that all procedures complied with ethical standards and that participants provided informed consent. The approval letter (No. INCAR-DG-DI-CI-206-2024) for publication is included in the Supplementary Materials and was granted in 2024.
2.4. Testing Procedures and Quality Control
Blood samples were collected in tubes without anticoagulants and for nucleic acid testing (NAT). The testing was conducted at the INCICH Blood Bank, an ISO 9001:2015-certified facility, adhering to national requirements as per NOM 253-SSA1-2012 [
15], enforced by COFEPRIS (Federal Commission for the Protection against Sanitary Risks). This regulation ensures compliance with transfusion-transmissible infectious agents’ detection and blood services licensing requirements. The INCICH Blood Bank adheres to high quality standards and strictly follows NOM 253-SSA1-2012 [
15], a national standard for the management of human blood and its components for therapeutic purposes.
2.5. Diagnostic Tests
Chemiluminescence assays were used for the initial diagnostic tests, performed on the Elecsys® analyzers by Roche Diagnostics, employing electrochemiluminescence (ECL) technology. These tests were conducted to detect antibodies for HIV, HBsAg, HCV, and syphilis. The assays are CE-marked and fully validated, and were conducted using the Roche Cobas 6000 and 8000 automated platforms. These automated systems integrate quality control mechanisms to ensure precise sample handling and reliable results.
Nucleic acid testing (NAT) was performed using the Procleix Panther System by Grifols, which detects nucleic acids of HIV-1, HBV, HCV, and Treponema pallidum (syphilis). These CE-marked assays are validated for blood screening, offering high sensitivity and specificity, especially in detecting early-phase infections and reducing the infectious window period. NAT testing was conducted using individual donor samples.
The tests used in our study were established by national regulatory standards to ensure compliance with the country’s legal framework and safety protocols. This information can be consulted online (
https://www.gob.mx/cofepris/acciones-y-programas/licencia-sanitaria-de-servicios-de-salud-modificacion, accessed on 1 October 2024). Additionally, our blood bank is certified under the NMX-CC-9001-IMNC2015/ISO-9001:2015 [
16] standard, reinforcing our commitment to regulatory and quality management systems. While alternative methods may exist, we adhered to the established regulations to ensure legal and safety compliance in Mexico.
2.6. Confirmatory Tests
If the diagnostic test results were reactive or doubtful, confirmatory testing was performed. For HIV, a Western blot (WB) using the Euroimmun Western Blot kit was applied, which detects HIV-specific antibodies (IgM/IgG) through electrophoretically separated viral antigens. For HCV, confirmatory testing involved the recombinant immunoblot assay (RIBA) using the Deciscan™ HCV PLUS system, which utilizes recombinant HCV proteins (NS3, NS4, Core antigens) through an indirect ELISA technique based on nitrocellulose strips. In the case of HBV, a neutralization test was used to confirm the presence of HBsAg. For syphilis, confirmatory testing involved Western blot (WB), targeting Treponema pallidum antigens to detect IgM antibodies.
Confirmation of results and follow-up involved contacting donors for a second sample collection within 2–4 weeks for HIV and HCV or 4–8 weeks for HBV to confirm initial results. Confirmed positive donors were referred for specialized medical care. The implementation of advanced technologies, such as the Procleix Ultrio Assay, allowed for earlier detection of infections, further enhancing transfusion safety.
2.7. Data Analysis
We conducted a secondary analysis of blood bank data to determine the prevalence of transfusion-transmissible infections in the blood donor population at INCICH. We estimated the overall prevalence and stratified it by sex. The incidence rates were calculated annually, and 95% confidence intervals and p values at a significance level of p < 0.05 were estimated. Additionally, we used a Poisson model to estimate the trend over the years 2012–2022. Finally, trends were calculated by sex and compared via Poisson prevalence rate differences to estimate the p value. The statistical analysis was performed using the STATA V13.0 software package.
4. Discussion
This study highlights the prevalence of TTIs among potential blood donors at the National Institute of Cardiology Ignacio Chávez (INCICH) over a ten-year period. The findings regarding the prevalence of HIV, syphilis, hepatitis B, and hepatitis C among blood donors shed light on the overall disease burden and inform strategies to enhance blood safety and donor screening. These results contribute to understanding the epidemiology of these infections in the general population and highlight the critical role of blood banks in identifying undiagnosed cases. Stringent donor selection, sensitive screening tests, and effective inactivation procedures are essential to minimize the risk of transfusion-transmitted infections and ensure a safe blood supply [
3,
5,
6,
13,
14,
15].
The use of nucleic acid testing (NAT) can significantly reduce the infectious window period for HIV, HCV, and HBV, leading to earlier detection and improved transfusion safety [
6,
12,
16]. However, the blood supply remains vulnerable to new and reemerging infectious agents [
5,
11,
13,
14]. Ongoing surveillance, education, and reporting of adverse events are necessary to monitor trends and guide policies to safeguard the blood supply [
13,
14,
15]. The study findings can inform the development of evidence-based strategies to enhance blood safety and donor screening practices at the national level. Concerted efforts are needed to increase voluntary blood donation, strengthen donor selection criteria, and implement advanced screening technologies to ensure the provision of the safest possible blood products for patients in need.
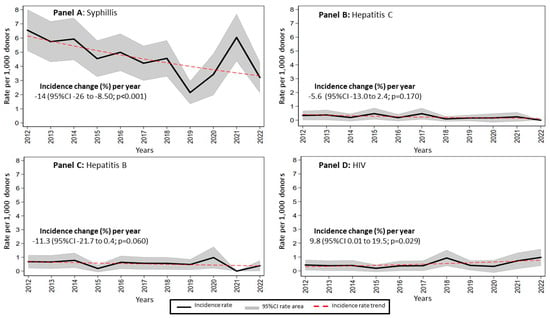
A large proportion of donors in this study were male (68.6%). Other studies also reported a larger number of male donors than female donors [
3,
17,
18]. This study revealed that syphilis had the highest prevalence among transfusion-transmissible infections at INCICH from 2012 to 2022, with a prevalence of 0.48% (95% CI: 0.44–0.52). The incidence of syphilis showed a significant annual decrease of −5.9% (
p < 0.001), although there were notable fluctuations, including a peak in 2021. This downward trend in syphilis incidence aligns with global data reported by the World Health Organization, indicating an overall decline in syphilis cases worldwide [
8]. However, the WHO has also reported periodic increases in syphilis incidence, particularly in certain regions. The fluctuations observed in our local data may be attributed to specific outbreaks or variations in the donor population at INCICH. Our findings are consistent with those of other regional studies in Mexico and the Latin American region with prevalence rates from 0.2 to 5.3 [
19]. This highlights the need for ongoing vigilance and targeted public health interventions to sustain and further improve the downward trend in syphilis prevalence among potential blood donors.
The prevalence of HIV was lower than that of syphilis, but showed a significant annual increase of 9.8% (
p = 0.029), peaking between 2019 and 2020. This contrasts with other studies reporting declining trends. Previous reports have reported prevalence rates ranging from 0.2 to 0.6%, 0.7%, and 1.2%, in Yemen, Sudan, and Mauritania, respectively, which are lower compared to the 1.18% observed in our study [
20]. The higher prevalence at INCICH may be attributed to the use of advanced detection methods, such as the Procleix Ultrio Assay, which enhances testing sensitivity [
6,
12,
16]. These results underscore the need for stronger HIV prevention and awareness efforts, particularly in areas with increasing rates. The variations between our findings and those from other studies emphasize the value of localized research to develop targeted and effective interventions.
The rise in HIV prevalence observed in our study likely stems from multiple factors beyond the sensitivity of detection methods. This increase in HIV and syphilis has been observed in other countries as well, often driven by high-risk behaviors like unprotected sex and intravenous drug use [
21]. Additionally, we need to consider if the worldwide COVID-19 pandemic exacerbated the situation, as limited access to healthcare services and delays in testing disproportionately affected vulnerable populations [
22,
23,
24,
25].
Fluctuations in syphilis prevalence, particularly the peak in 2021, could be linked to social behavior changes during the pandemic. Healthcare service disruptions and limited testing access may have caused delayed diagnoses, resulting in temporary spikes in cases once services resumed [
26,
27,
28].
Comparing our findings to research from countries like Brazil and Argentina, where early vaccination and screening programs have been implemented, may offer further insights. These countries have also faced fluctuations in HIV and syphilis rates due to disparities in access to preventive care, economic challenges, and educational gaps [
29,
30,
31]. This underscores the need for comprehensive public health strategies that address education, prevention, and screening to combat the rise in HIV and the variations in syphilis cases. Ongoing collaboration with regional and global studies will be crucial for understanding and addressing the underlying causes of these trends [
32,
33].
Hepatitis C showed a relatively stable incidence trend, with a slight annual decrease of −5.6% (
p = 0.170), maintaining low rates throughout the study period. This stability aligns with global efforts to control hepatitis C through improved screening and treatment protocols. The slight decline observed in our study, though not statistically significant, is nevertheless encouraging and suggests that current strategies to combat hepatitis C may be effective. Our data are consistent with other studies that reported low and stable hepatitis C rates, emphasizing the importance of continuing these effective public health measures [
11,
20,
34,
35]. Future research should explore ways to further reduce the prevalence of hepatitis C and address any emerging risks, such as identifying any potential new transmission routes or populations at greater risk. Additionally, exploring new diagnostic tools, treatment options, and prevention strategies could help drive the incidence of hepatitis C even more effectively.
The prevalence of hepatitis B showed a consistent downward trend in our study, with an annual decrease of −11.3% (
p = 0.060), reflecting the success of vaccination and screening efforts. Hepatitis B prevalence among donors at INCICH was 0.02% (95% CI: 0.01–0.03), notably lower than in higher-prevalence countries like India, where it reaches 0.36% [
23,
36]. This indicates that the vaccination strategy against hepatitis B in Mexico has been effective. While differences in vaccination coverage and demographic factors may contribute to variations between countries, our data underscore the importance of continuing preventive efforts to sustain low hepatitis B rates.
In Mexico, the hepatitis B vaccination was first introduced into the national immunization schedule in 1999, initially targeting infants [
37]; this program was later expanded in 2001 to include adolescents aged 10–19 years who had missed earlier vaccination opportunities. Given that our study focused on adults over 18 years old, most of the participants would have been born before these vaccination efforts were widely implemented [
38,
39]. This context helps explain the observed low prevalence of hepatitis B, suggesting that the vaccination programs for younger populations, along with robust screening measures, have played a crucial role in controlling the transmission of the virus. Furthermore, the use of HBsAg testing in our study allowed us to detect both acute and chronic infections, emphasizing the importance of early detection in curbing the spread of hepatitis B [
38,
39].
The United States, Brazil, Mexico, Argentina, and Chile were among the first to adopt vaccination programs as a strategic intervention to lower hepatitis B prevalence. In contrast to countries like India, where coverage and access remain uneven, the consistent application of vaccination policies in Mexico, including catch-up initiatives for adolescents, has likely contributed to the observed lower rates. Slight variations in our data compared to other studies may stem from differences in population demographics and vaccination reach. Nonetheless, our findings highlight the ongoing need for vaccination efforts and early detection to sustain low hepatitis B incidence. Future strategies should prioritize high-risk populations and ensure comprehensive blood donor screening to further minimize the residual risk of transfusion-transmissible infections.
In this study, NAT was performed using individual donor samples (ID-NAT), a method that provides higher sensitivity compared to pooling. ID-NAT allows for the detection of viral infections at lower viral loads, significantly reducing the infectious window and increasing the safety of blood products [
40,
41,
42,
43]. Pooling samples, while cost-effective, can dilute viral presence, potentially missing early-stage infections due to lower sensitivity [
41]. Studies comparing these two approaches demonstrate that ID-NAT is superior in detecting early infections, particularly for HIV, HBV, and HCV, where viral loads may be minimal during the initial stages [
44,
45,
46]. The use of ID-NAT in our study ensured that even low-level infections were identified, which is crucial for maintaining the highest safety standards in transfusion medicine. Although pooling can be logistically simpler and more economical, the increased sensitivity of ID-NAT justifies its use, particularly in high-risk settings where early detection is paramount. These findings align with previous studies that highlight the advantage of ID-NAT in improving transfusion safety by minimizing the risk of undetected infections.
4.1. Limitations
One of the main limitations of the secondary nature of the data analysis is that certain variables, such as detailed behavioral risk factors, were not available. Despite the limitations, the number of individuals included in this study and rigorous screening protocols employed at INCICH increased the reliability of our findings.
4.2. Recommendations for Future Research
Future research should focus on identifying and addressing barriers to blood donation among populations with high-risk factors for TTIs. Additionally, longitudinal studies can provide deeper insights into the effectiveness of intervention strategies over time. Enhancing public awareness and education regarding the importance of blood donation and safe practices could also contribute to reducing the prevalence of these infections.