1. Introduction
Dental caries is a chronic microbial illness affecting both children and adults worldwide and is one of the major oral diseases [
1,
2,
3]. The tooth surface becomes colonized (biofilm) by the bacteria that cause dental caries. The complex tooth structure is harmed by the presence of fermentable carbohydrates like fructose and sucrose [
4,
5].
Streptococcus and
Lactobacillus bacteria not only cause tooth decay but also play an important role in the progression of tooth decay [
6]. The most prevalent bacteria in saliva and tooth plaque,
Streptococcus mutans, is connected to initial caries [
7].
Lactobacillus species comprise 1% of the oral microbiota [
8].
Lactobacillus casei is essential because of its caries-forming properties.
Lactobacillus is acidogenic, and unlike their cariogenic partner,
S. mutans, they alone do not adhere to the tooth surface efficiently. However, with the presence of
S. mutans and the additional primary colonizers, their capacity to establish on tooth surfaces can be substantially augmented, though variations exist among different species. Therefore, they are more common in advanced caries lesions [
9].
There is a global need for safe, effective, and economical alternative preventive treatment options due to the rising incidence of oral diseases, particularly in developing countries, the resistance of pathogenic bacteria to currently used antibiotics and chemotherapeutics, and opportunistic infections in patients with compromised immune systems [
5,
10]. Despite the few commercially available therapeutic agents, these substances can change the oral microbiota and have unfavorable side effects such as vomiting, diarrhea, and tooth discoloration [
11]. Moreover the development of drug resistance against currently used antibiotics by various bacterial species that cause oral diseases is seen as a significant risk [
12]. For example, it was determined that
S. mutans strains in samples taken from fifty patients with dental caries showed high resistance to amoxicillin, ampicillin, erythromycin, tetracycline, and penicillin, and these strains showed 80% resistance to more than three antimicrobial agents [
13]. Furthermore, a cross-sectional study on bacteria isolated from dental caries reported that
S. mutans,
Streptococcus epidermidis,
Streptococcus oralis and
Lactobacillus acidophilus strains showed high resistance to penicillin by 82.2%, tetracycline by 88.4%, imperium by 100% and penicillin by 82.5%, respectively [
14]. For this reason, new approaches continue to search for alternative products to prevent dental caries [
15].
DNA is the pharmacological target of many drugs currently in use and used in clinical trials [
16]. This is because DNA has two main functions in the cell: replication and transcription. Replication and transcription are vital for cell survival, reproduction, and the proper functioning of the entire body. Drugs that interact with DNA cause the inhibition of DNA. In other words, it inhibits replication or protein synthesis and leads to cell death. Therefore, DNA inhibition is essential for antitumor and antimicrobial agents [
17]. In recent years, research on the identification of molecules interacting with DNA has received interest as a novel approach, and its application in industries, including biotechnology, nanotechnology, and the pharmaceutical sector, has been stressed [
18].
For thousands of years, plants have been utilized as a traditional medicine for treating and preventing numerous ailments in many different parts of the world [
19]. According to the World Health Organization (WHO), due to their accessibility, affordability, and lack of side effects, herbal medications are preferred by around 80% of the global population for the prevention of various diseases [
20,
21]. Plants are primarily used in dentistry to help prevent tooth decay and gum disorders due to their antibacterial effects [
22,
23]. In this respect, natural bioactive substances obtained from plants are currently used as additives. Among them, alkaloids, polyphenols, flavonoids, tannins, essential oils (EOs), terpenes, and terpenoids, as secondary metabolites, exhibit antimicrobial activity through different mechanisms of action [
24]. For example, alkaloids, which are investigated as a natural antibiotic type, are nitrogen-containing heterocyclic compounds. They have significant advantages such as structural diversity, broad antibacterial spectrum, rare side effect profiles and low tendency to trigger drug resistance. Alkaloids show activity through mechanisms of action such as inhibition of bacterial cell wall synthesis, metabolism, nucleic acid and protein synthesis or alteration of cell membrane permeability [
25]. Polyphenols with a wide structural diversity can show antimicrobial activity at the cellular level by damaging the cell wall, disrupting the cell membrane structure and function, inducing oxidative stress, inhibiting macromolecule synthesis, disrupting energy metabolism and triggering apoptosis-like death mechanisms. Moreover, polyphenols are known to show antibiofilm properties by affecting the c-di-GMP signaling system or quorum sensing system [
26]. On the other hand, EOs derived from plants have drawn more attention recently [
27].
EOs are one of the plant extracts used for centuries to prevent various medical and dental issues [
27,
28,
29]. They are known for their bactericidal, sedative, virucidal, anti-inflammatory, fungicidal, spasmolytic, local anesthetic, and analgesic properties. The presence of complex chemical structures consisting of several groups, such as terpenoids and terpenes, aromatic and aliphatic components, all characterized by low molecular weight, may explain their successful bacteriostatic and bactericidal effects [
30]. Interestingly, some of the research has proven that EOs can be beneficial against multidrug-resistant bacteria [
31,
32]. Among the plants investigated for the antimicrobial effects of EOs are cinnamon bark EO and orange EO. It is suggested in the literature that the components in cinnamon EO and cinnamon extracts may be beneficial in preventing caries and periodontal diseases with their antimicrobial activity against oral pathogens. Furthermore, it is known that cinnamon EO and cinnamon extracts contain cinnamaldehyde as the major component, and this compound has antibacterial properties. Therefore, more research should focus on the clinical use of oral care products containing cinnamon EO [
33]. Moreover, it has been determined that EOs obtained from orange peel have a strong antimicrobial effect on various pathogens. This effect is thought to be due to D-limonene, the main component of the EO [
34]. In addition, it was stated that these EOs obtained from orange peel have antimicrobial and antimycotic properties, which have a significant potential for use in mouthwash [
35,
36].
Despite the antibacterial activities of EOs, the hydrophobicity, volatility, toxicity and pungent odor of EOs limit their direct use [
37,
38]. Nanoparticle dosage forms, which can solve the stability problem of EOs, mask their pungent odor, and contribute to increasing bioavailability, can overcome this situation [
39]. Nanoparticles have attracted great interest due to solving the toxicity problem of active ingredients, drug targeting efficiency, increased bioavailability, and biodistribution [
40]. In addition, metal, metal oxide, calcium fluoride, chitosan and PLGA (poly-lactic-co-glycolic acid) polymeric nanoparticles are actively used as antimicrobial additives in dentistry. These nanoparticles perform effective interactions with bacterial cells due to their high surface area-to-volume ratio and high charge density [
24]. Here, PLGA has received Food and Drug Administration (FDA) approval for its biodegradability and biocompatibility. It is the best-defined biomaterial available for drug delivery in terms of design and performance [
41].
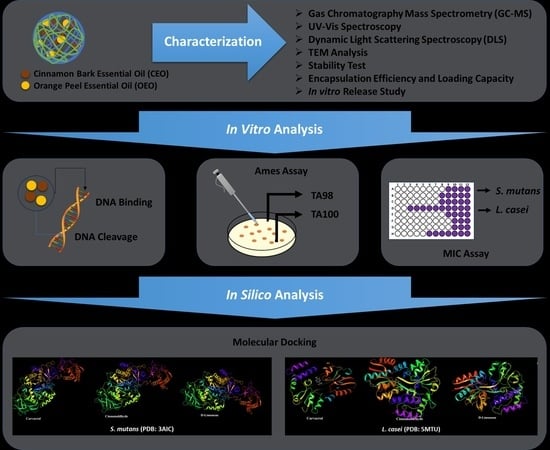
In our study, formulations in PLGA nanoparticle dosage form were synthesized by using cinnamon bark EO (CEO), orange peel EO (OEO), and the combined state of these two EOs (OEO-CEO). Characterization studies of the developed PLGA nanoparticles were carried out with various spectroscopic (UV–Vis spectroscopy, dynamic light scattering spectroscopy (DLS)) and transmission electron microscope (TEM) imaging methods. The antibacterial activity of the developed formulations was investigated on S. mutans and L. casei strains by in vitro and in silico methods. In addition to antibacterial studies, the interaction of EOs with DNA was also evaluated since the interaction of antibacterial agents with DNA is essential for antibacterial activity.
4. Discussion
To evaluate the average particle size, PdI, and zeta potential of blank PLGA and EO-loaded PLGA nanoparticles in our work, the commonly used DLS method was used [
43,
61]. The results were compared with other studies in the literature. Priyadarshini et al. (2018) synthesized clove oil-loaded PLGA nanoparticles for dental applications. They reported that blank nanoparticles had an average particle size of ∼127.84 ± 18.46 nm, while 10 and 25 mg oil-loaded nanoparticles had an average particle size of 165.37 ± 22.53 and 237.63 ± 13.68 nm, respectively [
65]. Phuangkaew et al. (2022) reported that nanoparticles obtained from amphiphilic quaternized chitosan for the prevention of dental caries have a hydrodynamic diameter of ~100–300 nm [
66]. Minhaco et al. (2023) synthesized curcumin-loaded PLGA nanoparticles for the treatment of endodontic biofilms and showed that blank and curcumin-loaded nanoparticles had an average particle size of 189.2 ± 13.630 and 247.6 ± 3.651 nm, respectively [
67]. In this context, it was concluded that the nanoparticles synthesized in our study have a suitable size range for dental applications. On the other hand, PdI values below 0.2 represent a narrow particle size range and monodispersity [
68]. Since the PdI value of the PLGA nanoparticles synthesized in our study was in the range of 0.032–0.078, it was concluded that they were monodisperse. Moreover, the zeta potential, which is used to measure the electrokinetic potential in colloidal systems [
69], is an important parameter for determining both the behavior of drug delivery systems [
70] and their colloidal stability [
71]. Gursu et al. (2022) synthesized cinnamaldehyde-loaded PLGA nanoparticles. The results reported that the zeta potential values of these particles ranged from − 3.54 to − 3.86 mV under three different storage conditions, and they showed good stability as higher negative zeta potential values increased the stability of the nanoparticles [
72]. Minhaco et al. (2023) synthesized curcumin-loaded PLGA nanoparticles to treat endodontic biofilms. They showed that blank and curcumin-loaded nanoparticles had zeta potential values of −0.935 ± 0.166 mV and −1.65 ± 0.355 mV, respectively [
67]. In this context, it was concluded that the nanoparticles synthesized in our study have a high zeta potential value compared to these studies in the literature.
In the commercialization phase, stability is strategically important regarding product shelf life. In this context, average particle size, PdI, and zeta potential values were compared to evaluate the stability of OEO-loaded PLGA, CEO-loaded PLGA, and OEO-CEO-loaded PLGA nanoparticles after lyophilization. The results showed that the DLS analysis results for the first day, first month, second month, and third month were close to each other, and there was no change that would cause significant stability problems. PVA used in PLGA nanoparticle preparation is known as a steric stabilizer [
73]. Here, steric stabilization is achieved by creating a repulsive force between the particles and droplets in the dispersion [
74] and preventing van der Waals attraction between nanoparticles [
75]. PVA used in the particle synthesis stage can be permanently bound to the nanoparticle surface thanks to the hydrophobic bond between partially hydrolyzed poly(vinyl acetate) groups and PLGA acetyl groups [
76]. Therefore, it was concluded that the nanoparticle formulations produced within the scope of our study remained stable for a long time after the lyophilization process and could be a product with a long shelf life since steric stabilization was provided by PVA bound to its surface in addition to the zeta potential.
High-level entrapment of the active ingredient in polymeric nanoparticles is important for developing effective nanoformulations. In this context, encapsulation efficiency and loading capacity parameters are a strategic approach to determine how much of the active ingredient is trapped in the polymeric envelope [
77]. Within the scope of our study, the encapsulation efficiency of the synthesized EO-loaded PLGA nanoparticles was carried out by the solvent extraction method [
44]. The results obtained in our study are consistent with the values obtained in the literature. Iannitelli et al. (2011) determined the encapsulation efficiency and loading capacity of carvacrol-loaded PLGA nanoparticles as 26% and 21%, respectively [
78]. Esfandyari-Manesh et al. (2013) synthesized PLGA nanoparticles loaded with anethole and carvone EOs by emulsification solvent evaporation and nanoprecipitation methods and determined that the highest encapsulation efficiency was 87.31 ± 5.84 and 68.21 ± 0.90, respectively, and the highest loading efficiency was 14.73 ± 0.82 and 13.64 ± 0.19, respectively [
79]. The encapsulation efficiency of the developed Bergamot EO-loaded nanoparticles was found to be between 28% and 84% [
39]. It was reported that PLGA nanoparticles loaded with
Cymbopogon citratus EO had an encapsulation efficiency of 73.29 ± 8.96 [
80]. PLGA-chitosan-folic acid nanoparticles containing
Artemisia vulgaris L. EO were produced, and the encapsulation efficiency was reported to be 99.79% [
43]. In another study, EOs obtained from
Boswellia sacra oleo gum resin were encapsulated with PLGA-PCL nanoparticles and the encapsulation efficiency was reported to be in the range of 27.04% ± 1.08 to 80.59 ± 3.37% [
81]. In this context, it was concluded that the EOs-loaded PLGA nanoparticles synthesized within the scope of our study have a high encapsulation efficiency and loading capacity. On the other hand, OEO-CEO-loaded PLGA nanoparticles’ encapsulation efficiency and loading capacity values of OEO are higher than those of CEO. Various parameters, such as polymer concentration in the oil phase, intrinsic viscosity, carboxylic terminal group, and molecular weight of polymers, greatly influence the encapsulation efficiency [
82]. Here, more hydrophilic drugs have lower affinity to the polymer, and this is an important parameter that reduces encapsulation efficiency [
83]. Based on our findings, the major components for OEO and CEO were determined to be D-limonene and cinnamaldehyde, respectively. Here, the limonene compound consisting of two isoprene units is completely hydrocarbon in structure and does not contain any polar groups [
84]. On the other hand, cinnamaldehyde is a compound containing two unsaturated functional groups of aldehyde and carbon-carbon double bond [
85], and this aldehyde group increases the polarity of the structure [
86]. Moreover, D-limonene has higher solubility in water compared to cinnamaldehyde (13.8 mg/L [
87] and 1.42 mg/mL [
88], respectively, at 25 °C). In this context, it was understood that this difference was due to the hydrophilicity and polarity of the major components in EOs and the better diffusion of OEO into the PLGA polymer compared to CEO.
The release of EOs from the nanoparticle depends on several factors, such as capsule wall thickness, affinity for the active compound PLGA, diffusion of the active compound through the polymer matrix, polymeric erosion, PLGA swelling and degradation [
89]. These parameters reveal the reason why the release profiles of EO-loaded PLGA nanoparticles in the literature are different from each other. Zhu et al. (2019) synthesized thymol-loaded PLGA microparticles and reported releasing 50% of thymol in 72 h [
90]. These results were closer to the amount of OEO (65.72 ± 0.57% within 48 h) released from PLGA nanoparticles loaded with OEO and CEO (44.27 ± 0.72% within 48 h) released from PLGA nanoparticles loaded with CEO. In another study with PLGA nanoparticles loaded with carvacrol, it was reported that 95% of carvacrol was released within 24 h [
78]. On the other hand, OEO-CEO-loaded PLGA nanoparticles released almost all of the OEO in 6 h, while the CEO released 74.94 ± 4.66% in 24 h. Manesh et al. (2013) developed anethole and carvone EO-loaded PLGA nanoparticles and reported that approximately 41% of anethole in 9 days and 50% of carvone in 4 days were released from PLGA nanoparticles [
79]. In this study, they reported that the release profile they obtained showed slow-release characteristics and that the diffusion of the loaded EOs occurred when the loaded essential oils passed through the PLGA polymer chains in the nanoparticle-controlled release system they prepared and passed from the polymeric matrix to the external environment. When the results we obtained in our study were evaluated, it was observed that a slow release profile similar to the literature was obtained when the release profile for OEO-loaded PLGA and CEO-loaded PLGA nanoparticles was examined. However, OEO-CEO-loaded PLGA nanoparticles determined that the release profile obtained from encapsulating two EOs with the polymer has a faster release profile than the PLGA nanoparticle-controlled system with a single EO load (
Figure 2e). The main component of CEO, cinnamaldehyde [
86], has a relatively polar property due to the aldehyde group it contains in its structure [
85]. Compounds containing aldehyde groups can form classical (OH····O) and weak (CH····O) hydrogen bonds with compounds containing carboxyl groups [
91]. Moreover, the carbonyl units in the PLGA structure can form hydrogen bonds [
92]. Therefore, CEO encapsulated in OEO-CEO-loaded PLGA nanoparticles may interact with the polymer and be released slower compared to OEO. On the other hand, since its main component, limonene does not contain a polar group [
84], OEO has a relatively more nonpolar property than CEO. Therefore, encapsulated CEO may partially polarize the polymer matrix and cause OEO-matrix compatibility to decrease, resulting in faster release of OEO, which has a higher nonpolar property.
Medicinal plants have been widely used in the development of new drugs from the past to the present. However, the use of plants in drug development causes safety concerns during preclinical evaluation. Genotoxicity analyses are extremely important in preclinical evaluation [
93]. Therefore, the Ames test, one of the main tools of genetic toxicology, has become part of a series of preclinical tests for the detection of the genotoxic potential of medicinal plants [
94,
95,
96]. When the concentrations of EOs used were evaluated by statistical analysis and Mortelman and Zeiger, it was determined that they had genotoxic effects on TA98 and TA100 mutant strains (
p < 0.05). In addition, at some concentrations of EOs, the number of colonies returned on the plate was very low. This indicates that the EO concentrations used are highly toxic [
48]. It was determined that EO-loaded PLGA nanoparticles did not have a genotoxic effect on
S. typhimurium TA98 and TA100 mutant strains according to both Mortelman and Zeiger and statistical analysis results (
p > 0.05). Our results clearly show that encapsulation of EOs with PLGA abolishes the genotoxicity of EOs.
Antibacterial activity tests are important methods for analyzing substances that can be used against disease-causing microorganisms [
97]. In this study, the effects of CEO, OEO, CEO-OEO, CEO- or OEO-loaded PLGA nanoparticles, CEO-OEO-loaded PLGA nanoparticles, and blank PLGA nanoparticles on
L. casei and
S. mutans, which negatively affect dental health, were determined. Based on the MIC results, both bacteria were found to be highly sensitive to CEO. Studies in the literature have determined that CEO is effective on both gram-positive and gram-negative bacteria [
98,
99,
100]. The active compound, cinnamaldehyde, is responsible for antibacterial activity, and the acrolein group (α,β-unsaturated carbonyl part) in this molecule has an important place for activity [
101]. Despite this, CEO-loaded PLGA nanoparticles were found to have a lower effect on bacteria than CEO. This was interpreted as due to the slow and controlled release of CEO from CEO-loaded PLGA nanoparticles [
89]. When the effectiveness of OEO on
L. casei and
S.mutans was examined, no activity was observed against
S. mutans. Pattnaik et al. (2010) evaluated the effectiveness of cinnamon oil, peppermint oil, cardamom oil and orange oil as antibacterial agents. The study found that orange oil was ineffective for gram-positive bacteria [
102]. Despite this, OEO was found effective on
L. casei and inhibited bacterial growth in MIC method. Moreover, it was found that OEO-loaded PLGA nanoparticles did not exhibit bactericidal effects on
L. casei and
S. mutans bacteria. On the other hand,
L. casei and
S. mutans were susceptible to CEO-OEO, with the antibacterial effect of CEO contributing significantly. Accordingly, it was determined that CEO-OEO-loaded PLGA nanoparticles were effective on these bacteria. When the minimum inhibitory concentration determined in the MIC method was examined, it was seen that CEO-OEO-loaded PLGA nanoparticles were effective on
L. casei and
S. mutans and had the same bactericidal effect. These findings were evaluated based on other studies in the literature. Limonene was determined to be the major component in EOs obtained from
Citrus reticulata Blanco peels, and a synergistic effect was observed on methicillin-resistant/susceptible
S. aureus as a result of the combined application of gentamicin/limonene and gentamicin/EOs [
103]. Application of gentamicin/D-limonene in combination showed a synergistic effect on multidrug-resistant
S. aureus and
E. coli strains, and MIC values decreased from 13.71 μg/mL to 4 μg/mL and from 30 μg/mL to 20.1 μg/mL, respectively [
104]. Echeverry-Chica et al. (2020) determined that nanoemulsions containing D-limonene-functionalized Ag nanoparticles showed a higher antibacterial effect compared to emulsions containing Ag nanoparticles or limonene alone [
105]. Motelica et al. (2023) reported that ZnO nanoparticles loaded with different EOs showed a higher inhibition zone compared to ZnO nanoparticles or EOs applied alone. They attributed this to sensitization by EOs, making the bacterial cell less resistant to ZnO nanoparticles [
106]. Motelica et al. (2024) developed hydroxyethylcellulose-based composite materials with ZnO nanoparticles, CEO-loaded mesoporous silica nanoparticles, and their combination. The combined material showed higher antibacterial activity against
S. aureus and
E. coli strains than CEO-loaded mesoporous silica nanoparticles loaded composite alone, providing a synergistic effect [
107]. Moreover, it is stated that the properties of the D-limonene compound, such as increasing the permeability of the antimicrobial substance or its lipophilic properties passing through the cell wall and changing the permeability of the cell membrane, may be related to this synergistic effect [
108]. In this context, our study has shown that the combined use of two EOs has a synergistic effect with OEO or CEO, thanks to limonene, the major component in its content, and is effective on both gram-positive and gram-negative bacteria.
Moreover, in our in silico results, it was determined that cinnamaldehyde, the main active component of CEO, and D-limonene, the main active component of OEO, bind to S. mutans and L. casei and that this binding occurs via the binding site to which carvacrol is found in the content of thyme oil, also binds, and that hydrogen bonds provide this binding. Moreover, it was determined by molecular docking analysis that the binding of cinnamaldehyde to S. mutans, i.e., its inhibition, was greater than D-limonene considering the docking score energy. However, the opposite was true for the binding and inhibition of L. casei, with D-limonene providing a more stable binding. It was observed as a result of this study that the main active ingredients of both EOs could be at least as effective as carvacrol in the content of oregano oil against these bacteria.
In our study, DNA binding activity of OEO and CEO was evaluated, and it was determined that both CEO and OEO showed a 6 nm red shift and 29.09% and 17.60% hypochromic effect, respectively, as a result of their interaction with CT-DNA. In spectral effects, the blank π* orbital of the molecule pairs with the π* orbital of the DNA base pairs, resulting in an energy decrease and a lowering of the π-π* transition energy. This is detected by the red shift of the absorption in the molecular DNA interaction. At the same time, the blank π* orbital is partially filled with electrons to reduce the probability of transition, resulting in hypochromism [
109,
110]. Hypochromism and red-shift are considered to be indicators of intercalative binding of compounds with DNA [
38,
111]. Compounds with clinical potential may interact with DNA by intercalation or groove linking [
112]. In this context, the results obtained show that OEO and CEO exhibit intercalative interaction with DNA and, therefore, may be effective agents for antibacterial activity.
DNA cleavage activity of OEO, CEO, CEO-OEO, OEO-loaded PLGA, CEO-loaded PLGA, and CEO-OEO-loaded PLGA nanoparticles was determined using pBR322 plasmid DNA by agarose gel electrophoresis [
113,
114,
115]. The main targets in DNA cutting are the phosphodiester bond, deoxyribose sugar, or nucleobases of DNA. It is possible to cut these important parts of DNA by hydrolytic or oxidative means. While hydrolytic cleavage occurs at the phosphodiester bonds of DNA, oxidative cleavage occurs at the deoxyribose sugar or nucleobases of DNA [
116]. In the study, pBR322 plasmid DNA was used for DNA-cutting activity. Circular plasmid DNA migrates relatively quickly in gel electrophoresis (Form I). If cutting occurs in a single strand of plasmid DNA, the supercoiled state of the plasmid relaxes and transforms into Form II. If both strands are cleaved, Form III is formed, a linear form that migrates between Form I and Form II [
117]. When these results were examined, it was interpreted that the reason for the lack of cleavage activity in CEO-loaded PLGA nanoparticles could be due to the DNA cleavage activity test system being completed in a short time of 3 h and CEO being released slowly from the particle (44.27 ± 0.72% in 48 h) and not showing any activity. On the other hand, it was thought that the cleavage activity of OEO-loaded PLGA nanoparticles and OEO-CEO-loaded PLGA nanoparticles could be due to the rapid release.