1. Introduction
Hepatocellular carcinoma (HCC) is a severe complication of chronic liver disease, particularly liver cirrhosis, that often leads to poor outcomes [
1]. It ranks as the third most common cause of cancer-related deaths globally [
2]. The Barcelona Clinic Liver Cancer (BCLC) staging system is the most widely recognized framework for guiding treatment in HCC patients [
3,
4]. For intermediate-stage HCC (BCLC-B), transarterial chemoembolization (TACE) is the standard treatment [
4]. While TACE has been shown to improve survival in BCLC-B HCC, it can also result in the deterioration of liver function, particularly in patients with a high tumor burden and limited hepatic reserve, which leads to unfavorable outcomes [
5,
6].
The Child–Pugh classification, commonly used to assess liver function reserve in cirrhosis, includes subjective elements, like ascites and hepatic encephalopathy [
7]. As a more objective alternative, the albumin–bilirubin (ALBI) grade, based on serum albumin and total bilirubin levels, has been proposed for evaluating liver function reserve across HCC stages [
7]. The ALBI grade is categorized into three grades: grade 1 (≤−2.6), grade 2 (>−2.6 to ≤−1.39), and grade 3 (>−1.39) [
7]. A recent study by Zhao et al. highlighted the superiority of the ALBI grade over Child–Pugh classification in predicting overall survival (OS) in HCC patients undergoing TACE [
8]. Given that serum albumin and bilirubin levels are affected by various non-hepatic factors, there is a growing need for more precise liver function evaluation models. Recent developments include scoring systems that integrate the ALBI grade for a more thorough assessment of hepatic function and improved prognosis prediction in HCC [
9,
10,
11].
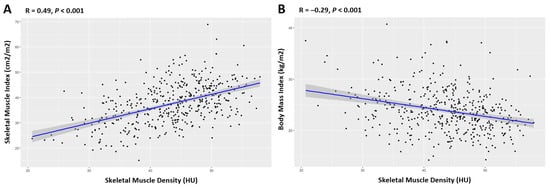
Skeletal muscle density (SMD) at the third lumbar (L3) vertebra, which is measured in Hounsfield units (HU) via computed tomography (CT) images, serves as a muscle quality indicator. Low SMD, or myosteatosis, signifies pathological fat accumulation in muscle tissue that can potentially lead to inflammation, insulin resistance, and adverse outcomes in cancer treatment due to inflammatory adipokines from intermuscular fat [
12,
13,
14,
15,
16]. Recent findings have linked myosteatosis with a diminished response to TACE and reduced survival in HCC patients [
17].
However, the combined prognostic effect of myosteatosis and the ALBI grade on clinical outcomes in HCC remains largely unknown. Therefore, this study aimed to assess the prognostic efficacy of preoperative myosteatosis and the ALBI grade and develop a robust prognostic score based on these factors to efficiently predict long-term outcomes for patients with unresectable HCC.
4. Discussion
Several studies have shown that both myosteatosis and the ALBI grade are associated with the prognosis of multiple cancers; however, most studies have addressed them individually. The correlations between myosteatosis and the ALBI grade and their combined prognostic values have remained unclear in HCC. In this study, we proved that myosteatosis and the ALBI grade were independent predictors of OS for patients with unresectable HCC undergoing TACE. Our combination of the Myo-ALBI grade and pretreatment clinicopathological data (the AFP level and up-to-seven criteria) demonstrated a modest performance in differentiating survival outcomes and exhibited superior prognostic ability for long-term survival compared to the Child–Pugh class and up-to-seven criteria. In summary, this study highlights the promising prognostic value of combining myosteatosis and the ALBI grade to assess HCC patients undergoing TACE in a clinical setting.
The exact mechanism through which the Myo-ALBI grade predicts the prognosis of HCC is not fully understood, but it likely stems from the integration of myosteatosis status with serum albumin and total bilirubin levels. Myosteatosis, characterized by the infiltration of fat into skeletal muscle, leading to reduced muscle quality and strength, is increasingly recognized as a poor prognostic factor in malignancies and is closely associated with tumor progression and mortality [
20,
21,
22]. Specifically, myosteatosis has been shown to contribute to a poorer response to TACE and an increased risk of mortality in HCC, more so than sarcopenia [
17]. This could be attributed to adipocytes in myosteatosis releasing inflammatory adipokines, which impair muscle blood flow and exacerbate insulin resistance. These changes may weaken the body’s immunity and promote cancer growth, leading to adverse treatment outcomes [
23,
24]. Furthermore, albumin and bilirubin levels play a pivotal role in predicting the prognosis of HCC, as they reflect liver synthetic function (albumin) and the liver’s ability to process waste (bilirubin). Changes in albumin and bilirubin levels can reveal the degree of liver damage and significantly impact outcomes for patients with HCC [
25,
26].
The Myo-ALBI grade, which encompasses both liver functional reserve, as indicated by albumin and bilirubin levels, and CT-derived muscle quality and nutritional status, as denoted by the presence or absence of preoperative myosteatosis, stands as a reliable tool for predicting outcomes. This integrated approach effectively identifies more at-risk HCC patients and provides a comprehensive assessment of prognostic factors. This is particularly crucial for patients with HCC undergoing TACE due to the treatment’s potential systemic effects on overall patient health. The prognostic accuracy of the Myo-ALBI grade was higher than either preoperative myosteatosis or the ALBI grade. Furthermore, patients with ALBI grade 3 and myosteatosis (Myo-ALBI grade 3) had a nearly fourfold increased risk of death from any cause compared to other groups. Patients with Myo-ALBI grade 3 had a median OS of 7.2 months (95% CI, 5.4–11.8), which is comparable to patients with BCLC-C HCC who received sorafenib treatment [
27]. Therefore, caution is advised when considering TACE as a treatment option for these patients. Alternative treatment options with more favorable safety profiles and better survival benefits should be considered in this group.
The strong associations between Myo-ALBI grade 3 and various poor prognostic features (such as older age, higher BMI, elevated INR, lower albumin, higher bilirubin, and higher tumor burden) (
Table 3) further validate this novel grading system. By combining myosteatosis and the ALBI grade, Myo-ALBI captures a broader spectrum of adverse factors than either component alone, potentially explaining its superior prognostic performance. The inverse relationship between Myo-ALBI grades 0 and 1 for several parameters highlights the complex interplay between liver function and muscle health in HCC. For instance, younger patients may progress to grade 1 earlier due to more aggressive disease, while older grade 0 patients may have developed compensatory mechanisms. The higher prevalence of HBV carriers in grade 0 could reflect earlier diagnosis through surveillance programs. Notably, the lower incidence of sarcopenia in grade 1 suggests muscle mass loss may precede changes in muscle quality (myosteatosis) in some patients. These observations underscore HCC’s complex pathophysiology and the value of Myo-ALBI as a comprehensive prognostic tool for clinical decision-making in HCC patients undergoing TACE.
In the context of BCLC stage B, TACE is the primary treatment for unresectable HCC patients [
4]. However, prognoses can vary among HCC patients with the same BCLC-B stage due to differences in tumor size, number, vascularity, and underlying liver function [
5,
6]. According to the latest BCLC system, BCLC-B HCC can be divided into three subgroups. Patients with diffuse, infiltrative, or bilobar disease are unlikely to benefit from TACE, and systemic therapy should be considered instead [
4]. However, there are no definite criteria for identifying BCLC-B HCC patients who should proceed directly to systemic treatment as their first-line therapy. In most major interdisciplinary treatment centers and clinical trials, HCC patients classified as Child–Pugh class A are often the primary focus [
28]. This reflects the selection of a patient population with relatively preserved liver function, which is crucial for optimal treatment outcomes. The up-to-seven criteria, which combines the number of tumors and the size of the largest tumor, is an effective tool to stratify BCLC-B HCC patients for appropriate treatment modalities [
11]. Patients within the up-to-seven criteria (limited tumor burden) benefit more from TACE, while those beyond the criteria should be considered for systemic therapies to improve survival outcomes [
29,
30]. Our Myo-ALBI nomogram was constructed by incorporating the Myo-ALBI grade, AFP level, and up-to-seven criteria, and it performed well in internal validation. When assessing overall survival in BCLC-B patients, the Myo-ALBI nomogram demonstrated higher predictive accuracy compared to non-Myo-ALBI nomograms, up-to-seven criteria, ALBI grade, and Child–Pugh class. Thus, the Myo-ALBI grade can improve the prediction of prognoses in patients with HCC undergoing TACE. In clinical practice, the Myo-ALBI grade can be used as a supplement to the Child–Pugh class or up-to-seven criteria to better stratify BCLC-B HCC patients and provide a more accurate basis for guiding postoperative follow-up and treatment.
While TACE primarily targets HCC, its efficacy and tolerability are linked to both liver function and overall patient health. The Myo-ALBI score, combining muscle quality (myosteatosis) and liver function (the ALBI grade), provides a comprehensive evaluation of a patient’s physiological reserve. This is particularly relevant for TACE due to its impact on treatment tolerability, recovery between sessions, ability to handle systemic effects, and potential influence on the tumor microenvironment [
4,
5,
6]. As demonstrated in our previous work [
17], myosteatosis is associated with poor TACE response and reduced survival. The underlying mechanism involves several factors, including inflammatory adipokines, impaired muscle blood flow, and insulin resistance. These factors can weaken immunity, promote cancer growth, and create a pro-tumorigenic environment, potentially influencing TACE effectiveness and tumor progression [
17].
The Myo-ALBI score represents a significant advancement in prognostication for HCC patients considered for TACE. Unlike our previous SMAART score [
30], which assessed patients after the first TACE session, the Myo-ALBI score is applied pre-TACE, allowing for more informed initial treatment decisions. Moreover, it uniquely incorporates myosteatosis, reflecting both hepatic and overall physical status. This comprehensive pre-treatment evaluation not only improves prognostic accuracy but also opens new avenues for personalized treatment strategies, potentially guiding decisions toward alternative or combination therapies in high-risk patients before TACE initiation.
Several limitations were present in our study. First, the retrospective design possibly introduced potential biases. Second, since the study was conducted at a single center, the generalizability of the findings may be limited, which underscores the need for larger-scale, multicenter randomized controlled trials to validate these results. Finally, the predominance of HBV infection as the cause of HCC in this Thai cohort may not reflect the etiology of HCC in other regions where HCV infection and alcohol abuse might be more prevalent. This regional variation could lead to differences in tumor imaging characteristics that potentially affect the applicability of our findings to broader populations.