1. Introduction
In recent years, the production of plastics has increased dramatically every year worldwide, reaching an extremely high production of 400 million tons [
1]. These versatile materials are widely used around the world for many applications, especially in packaging, due to their bio-inertness, light weight, transparency, ductility, and low cost. This high increase in production and the use of these products makes their recycling and minimization a key concern to society, since only 9% of plastic is currently recycled [
2]. Specifically, plastic waste generation was 6300 Mt between 1950 and 2015, and around 5000 Mt was discharged into the environment [
3]. In 2019, around 22 Mt of plastic leaked into the environment, with 12% represented by polymer particles less than 5 mm in size [
2]. This has resulted in the buildup of a massive volume of waste in the environment.
Plastics with a size of less than 5 mm are called microplastics (MPs) [
4], which originate from domestic and industrial use (especially in cosmetics or pharmaceuticals) or from the degradation of the large amount of non-recycled and accumulated plastics. Their small size facilitates their dispersion in the natural environment including air, water, and soil. These MPs break down even further into nanoplastics (NPs), which include nanoparticles from 1 to 1000 nm [
5], which further facilitates their dispersion and presence in ecosystems. Apart from size, MPs and NPs present different physical and chemical characteristics like different light diffraction patterns, sedimentation and buoyancy, or the proportion of surface molecules [
6]. A study from Gigault et al. showed that NPs are even more toxic and harmful to ecosystems than MPs [
6]. In a plant model, for example, NPs allow for greater bioavailability compared to larger micrometer particles [
7], and in a Daphnia model, NPs induced lower egestion and reduced feeding rates [
8]. For example, it has also been reported that the internalization rate of 100 nm NPs in blue mussel larvae is 6 to 10 times higher than that of 2 μm MPs, resulting in a higher malformation rate [
9]. However, the detection of NPs is much more complex and the adverse effects of NPs have been less studied.
In the last 10 years, the number of published studies on the distribution, properties, and effects of MPs and NPs in the environment as well as their impact on ecosystems and human health has rapidly increased. MPs and NPs are currently widely distributed in the environment and their presence in different environmental compartments such as marine waters, lakes, rivers, alpine snow, sediments, sand, field samples, food, biota, or in the air has been detected in several studies [
10,
11,
12,
13,
14,
15,
16,
17]. Remarkably, NPs and MPs have been found across a range of living organisms that include plants, animals, and humans [
18,
19,
20,
21].
Li et al. reported the uptake of MPs and NPs by plants from plastic-contaminated soils while demonstrating their translocation and accumulation [
22,
23]. One pathway of exposure in animals, either marine or terrestrial, is ingestion. Biofilms in MPs ingested by marine animals ultimately enter other trophic levels [
24,
25,
26,
27].
In general, plastic polymers are biochemically inert due to their large molecular size and are not considered hazardous to the environment. However, polymerization may be incomplete, and polymers may have some residual monomers, which modify the properties of the polymer and increase its hazardousness [
28]. In addition, polymers may contain chemical additives and unwanted substances such as reaction by-products or impurities including low molecular weight polymer fragments, oligomers, catalyst residues, polymerization solvents, surfactant, and processing additives or final product additives [
29]. In some cases, the polymerization chemicals, additives, or raw materials mentioned are considered even more hazardous than the monomers [
30]. These compounds usually have a low molecular weight and can migrate to other substances such as packaged foods [
29]. It was noted in the hazard classification presented by Lithner and colleagues that 29% of the 55 tested polymers were severely hazardous to health, with examples such as cancer inducers or genetic defect inducers, which included polyurethane, polyacrylonitrile polymers, and PVC, and 56% of them had milder toxicity, which included allergic skin reactions or organ damage [
30]. In addition, plastics can accumulate organic chemicals outside the manufacturing process (e.g., polycyclic aromatic hydrocarbons or polychlorinated biphenyls) and trace metals (e.g., lead, copper, cadmium or mercury) from the environment [
31,
32] along with biofilms that form on the plastics.
Several publications have shown the evaluation of toxicity and the chemical behavior of MPs in particular [
33,
34,
35], and many scientists are investigating MPs and NPs with regard to their association with reproductive abnormalities and cardiovascular risks in human and animal cells [
36,
37]. A study by Pencik et al. nicely summarizes the status of the microbiological toxicity studies in aquatic species to date, while highlighting the caveats and needs of MP and NP studies [
38]. The authors emphasize that many studies use a higher and unrealistic dose of MPs to assess toxicity [
38], and that to conduct an in vitro toxicity study accurately, the first thing we need to do is quantify the current plastic levels present in the biota. The adverse effects of persistent MPs and NPs that accumulate over time deserve further studies.
Here, we show the prospective results of plastic accumulation in humans and mice by means of a novel method of nanocytometry previously described in our laboratory [
20]. By analyzing different human tissues and samples, it became possible to analyze new information about the different entry paths and their accumulation in our organism and whether they could be related to the different pathologies analyzed. Finally, based on the detection of NPs in mice bred under controlled air filtration, we hypothesize that the inhalation of airborne NPs has an important impact on their accumulation in the organism.
2. Material and Methods
2.1. Human Samples
Lymph node biopsies, bronchoalveolar lavage fluids, cerebrospinal fluids, pleural fluids, ascitic fluids, urine, and peripheral blood were analyzed. Lymph node biopsies (n = 44) were obtained by fine needle aspiration or core needle biopsy and collected in RPMI 1640 medium. Bronchoalveolar lavage fluids (n = 41) were collected and resuspended in saline. Urine (n = 26), cerebrospinal (n = 24), pleural (n = 18), and ascitic (n = 7) fluids were collected in glass tubes. Peripheral blood samples (n = 434) were collected by venipuncture in EDTA tubes and stored at room temperature. All samples were obtained from the Hematology Dept. of the Hospital Universitari Germans i Pujol (HUGTIP; Badalona, Spain). Pediatric patients with type 1 diabetes (T1D), from diagnosis to 12 months of disease evolution (n = 89), were recruited from the Pediatrics Dept of the Hospital Universitari Germans Trias i Pujol (HUGTIP) and Parc Taulí University Hospital (Sabadell, Spain). All patients met the American Diabetes Association classification criteria for T1D. Multiple sclerosis patients were recruited from the HUGTIP Neurology Unit.
For the peripheral blood study, patients were chosen according to their pathology, while the other tissue samples were chosen according to their availability. Adult controls were blood donors who accepted the study. Control samples from pediatric subjects (based on sex and age) were obtained simultaneously with the diagnosis of children with T1D. Inclusion criteria were being between 4 and 18 years of age and having a normal body mass index (BMI) according to the Spanish pediatric cohort BMI growth chart [
39]. Exclusion criteria were being under immunosuppressive or anti-inflammatory treatment, the presence of other autoimmune diseases, type 2 diabetes, pregnancy, compromised renal function, or liver disease. The pediatric controls were mainly children who came to our center (Hospital Universitari Germans Trias i Pujol, Badalona, Spain) for minor surgery without previous inflammation (for example, phimosis) or for blood tests requested by their pediatrician (suspicion of metabolic disorder later ruled out). In any case, the professionals at the Pediatrics Service confirmed that the controls met the aforementioned inclusion criteria.
2.2. Mice
Wild-type NOD mice and C57BL/6 mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and Charles River Laboratories (Wilmington, MA, USA) respectively, and then kept in the Animal Facility of the Centre de Medicina Comparativa i Bioimatge de Catalunya (CMCiB, Badalona, Spain) under specific pathogen-free conditions. Both strains were subjected to a 12-h dark/12-h light cycle and controlled temperatures between 19 and 23 °C with 40–60% humidity and fed with ad libitum access to acidic water at pH 5 and Teklad Global 18% Protein Rodent Diet (Harlan, Indianapolis, IN, USA). In all cases, 39-week-old mice were used (n = 40; 12 male and 28 female).
2.3. Sample Preparation and Flow Cytometry
All samples were manipulated in a BSL2+ environment to prevent airborne contamination. Eppendorf Safe-Lock
® tubes (Hamburg, Germany) were used in this study based on high chemical and thermal resistance. For the peripheral blood and urine samples, 20 µL of blood was incubated in 1% aqueous potassium hydroxide (KOH) (Merck, Rahway, NJ, USA) (
w/
v, 1000 µL final volume) in a heat block at 60 °C for 10 days [
20]. In addition, lymph nodes, cerebrospinal fluid, pleural fluid, ascitic fluid, and bronchoalveolar lavage fluid were lyophilized in a lyophilizer (B. Braun Biotech, Melsungen, Germany) before their dry weight was recorded. Following lyophilization, samples were incubated in 1% aqueous KOH in a heat block at 60 °C for 10 days.
All specimens were prepared in triplicate by diluting 20 µL of the digested sample in pyrogen-free water (1000 µL final volume) and stained with 2 µL of Nile red (Sigma-Aldrich, Burlington, MA, USA; 0.1 mg/mL stock solution). After a 15 min incubation at room temperature and light protected, samples were acquired on the Attune™ NxT Flow Cytometer (Thermo Fisher Scientific, Waltham, MA, USA) at the lowest possible sample rate (12.5 µL/min) with the H-pulse parameter and the violet-side scattering was collected using the Attune™ NxT Violet Side-Scatter Filter Kit. An exhaustive rinse to minimize carry over with filtered deionized water was performed between each sample. In addition, a thorough cleaning of the flow cell was performed prior to and after sampling (Hellmanex® III, Attune NxT Flow Cell Cleaning Solution, Cat# A43635). Nile red was excited at 561 nm and its emission was collected with 585/16 BP in the YL1 detector. The submicron particle size reference kit consisting of 0.2 μm, 0.5 μm, and 0.8 μm beads (Bangs Laboratories, Inc., Fishers, IN, USA) was used for calibration. For high-resolution brightfield imaging, the Attune™ CytPix flow cytometer, which combines acoustic focusing with a high-speed brightfield camera for simultaneous high-throughput flow cytometry, was used. Automated image analysis with the Attune Cytometric Software (v. 7.1) translates event features into distinct morphometric parameters that can be combined with standard fluorescence and scatter parameters.
2.4. Quality Control
The sampling and processing of biological tissues involves the use of plastic materials and therefore may involve potential contamination. Blood collection tubes were made of durable polyethylene terephthalate (PET), and both Eppendorf Safe-Lock microtubes and 75 × 12 flow cytometry tubes were made of polypropylene (PP). To rule out any contribution of the sample collection and processing to the final quantification, all materials used in this study were CE-IVD certified and evaluated for trace amounts of plastic. For sample collection, RPMI 1640, saline and EDTA were tested, and for sample processing, 1% KOH and pyrogen-free water were tested by incubating 1 mL of each reagent with Nile red as described. Plastic measurements obtained from the pyrogen-free water, EDTA tubes were consistently lower than 39 events/μL, while those from aqueous KOH were consistently lower than 55 events/μL. Pyrogen-free water was tested daily to maintain thorough control of the cytometer and materials (
Supplementary Figure S1). In addition, the potential variation in NR fluorescence obtained from different stock solutions used over time was always monitored using blank beads to ensure fluorescence calibration.
2.5. Ethics
For the human samples, the study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of IGTP (protocol codes: PI-22-209; PI-22-266; PI-23-003; date of approval 4 October 2022; 18 November 2022; 19 December 2022).
For the mouse samples, this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Generalitat de Catalunya. The procedures carried out with animal models were authorized by the Animal Experimentation Ethics Committee of the CMCiB and IGTP and by the Generalitat de Catalunya, and followed the principles outlined in the Declaration of Helsinki for animal experimental investigation. All of the conducted protocols followed the principles of the 3R, prioritizing the welfare of animals used in research.
2.6. Statistics
All measurements were performed in triplicate, and a quadruplicate was added if any of the results showed discrepancies. Graphs were generated using Prism software (v.9). The median, mean, standard deviation, and first and third quartiles were calculated for a total of 594 human subjects and 40 mice included in this study. The absolute number of NPs in each category was compared using nonparametric analyses with SAS software (v. 9.4) such as a general linear model and Tukey–Kramer adjusted p-values for differences in least squares means and the Wilcoxon rank sum test when comparing two variables. A p-value < 0.05 was considered statistically significant.
3. Results and Discussion
This new study conducted in Barcelona (Spain) shows that the presence of nanoplastics is ubiquitous in the human body. We analyzed more than 500 samples from patients in the metropolitan area of Barcelona and found NPs in all of them. The research shows that these plastic nanoparticles are disseminated in large numbers within our body. Our study went a step further by demonstrating different dispersions of NPs in peripheral blood, which may show an impact on the immune system. Further research is needed to understand the type, origin, and possible health effects of nanoparticles.
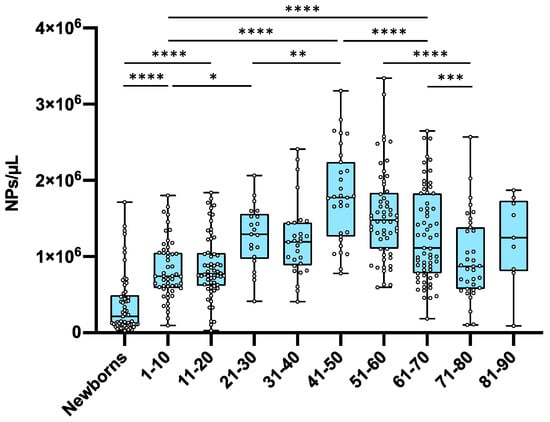
In this study, the presence of NPs in newborns was investigated based on previous studies in the field of MPs in the human placenta [
40,
41]. We analyzed blood samples from 61 newborns and detected the presence of NPs in each case, suggesting in utero or early life exposure, and subsequent accumulation and later decline warrant further investigation. The studied newborns exhibited the lowest NP levels compared to all of the other age groups,
p-value < 0.0001. NP accumulation with age started to increase progressively until it reached a peak at the age of about 50 years old. After the age of 50 years, accumulation tended to decrease progressively in each decade (
Figure 1). As for sex, no differences were observed among adults (
Figure 2).
There were no differences in pathology among pediatric patients with type 1 diabetes or idiopathic nephrotic disease compared to the controls (
Figure 3). Adult pathologies of chronic lymphocytic leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, and multiple myeloma were also all similar to the controls. Notably, lung cancer patients had a significantly reduced concentration of NPs (
p-value < 0.0001). Here, the effect of increasing age in the accumulation of NPs was, again, significantly related, with the neonates, pediatric, and adult controls all having significantly increasing NP levels with age. When grouped by disease type of the same age group, no differences in accumulation were observed (
Figure 4A). When these six adult diseases were combined on a global basis, the pathology as a whole tended to have a lower presence of NPs compared to the controls, with a
p-value of 0.1210 (
Figure 4B).
Patients with T1D were analyzed according to their disease stage [
42]: onset, partial remission (PR), non-partial remission (non-PR; eighth month in patients who had not achieved remission), and long-standing (more than 12 months of disease evolution). Although the entire cohort of T1D patients showed no difference from the controls (
Figure 3), a decreasing trend of NP accumulation was observed in the different stages of T1D (
Figure 5). Specifically, newly diagnosed patients showed a tendency to accumulate a higher concentration of NPs compared to patients who had suffered the disease for a longer period of time (stabilized disease), even if it was not significant.
Smoking is a harmful habit for health, as it is the main cause of many diseases. Tobacco smoke, classified as carcinogenic, is a toxic mixture of some 5000 chemical substances and compounds. Its inhalation transports these harmful particles and chemicals to the lungs, which can end up in the blood, especially in the case of nanoparticles, which are deposited in the alveoli and penetrate the blood system [
43]. It has been shown that 8.43 × 10
9 particles of particulate matter can be inhaled in the first second of inhaling a cigarette [
44]. On this basis, we wanted to compare NPs in the blood of smokers and nonsmokers. Data on smoking in lung cancer (LC) patients were available and a comparison was made between former smokers and current smokers. Unfortunately, in our group, patients who had never smoked were not available. Active smokers tended to have a higher NP accumulation than former smokers, although not significantly (
p-value = 0.2064) (
Figure 6).
To further our understanding of the distribution and accumulation of NPs, we analyzed the following human tissues and samples: lymph nodes, bronchoalveolar lavage (BAL), urine, pleural fluid, ascites, and cerebrospinal fluid (CSF). For more information on the tissue samples, please see
Supplementary Table S1 (Patients’ characteristics). Urine showed levels 37 times lower than those of peripheral blood (
Figure 7A). Considering NP accumulation in relation to dry tissue weight, the highest values were those of peripheral blood and the lymph nodes. BAL presented significantly 2-fold lower levels, but with a markedly greater dispersion among samples. Pleural fluids presented levels similar to those of BAL and a significantly higher concentration of NPs than ascitic fluids, being 6 times lower than that of PB. Finally, the CSF samples presented a 14-fold decrease with respect to PB (
Figure 7B). It should be noted that the sample size was very different between groups, with the blood group being much larger than the others. Therefore, these results are exploratory. In general, urine appeared to be the biological fluid with the lowest accumulation of NPs, probably related to the higher dilution rate and the nanofiltration function of the kidneys.
Plastic pollution is permeating our planet on a global scale. Contrary to the common literature, inhalation could be the main route of plastic accumulation in living beings, so we decided to compare our cohort from the Barcelona Metropolitan Area with mice kept in a specific pathogen free (SPF) laboratory guaranteed to be free of particular pathogens. Ventilated animal storage systems protect the animals but do not contain hazardous aerosols, which can be released from infected animals. In addition, the pre-filtered air is biologically cleaned by a HEPA filter that stops all particles and biological matter down to 0.3 microns to protect the animals. The NP levels were evaluated in two mouse strains, NOD and C57BL/6, and the blood of mice presented a concentration 3 times lower than that of humans in both cases, with a
p-value < 0.0001 (
Figure 8). Furthermore, there was no difference between strains (
p-value = 0.9550), highlighting the research interest in understanding the role of air in NP accumulation within the human body. Interestingly, the blood levels of NPs found in mice provided results comparable to those obtained in newborns after a screening blood test, usually after sampling 48–72 h after the birth of the baby, suggesting that the placental barrier acts similarly to the HEPA filters installed in SPF laboratories.
Laboratory mice have long been widely used as model organisms for testing hypotheses and therapies aimed at understanding disease mechanisms in humans. However, the most relevant experimental scenarios must integrate a very precise knowledge of the similarities and differences at many levels between mice and humans. Considering the important differences between the two species in terms of genetic, physiological, and immunological aspects, much more research is needed to understand how these differences impact on whole organism function and disease mechanisms that would be relevant to the accumulation of plastics in humans. Although the newborns and mice provided comparable levels of plastic accumulation, the interpretation of these results should be made with caution. Nevertheless, for the first time, we provide preliminary data on this important topic, which may help guide further research in this area.
The analysis of nanoplastic particles usually involves the use of advanced imaging techniques such as scanning and transmission electron microscopy. However, although electron microscopy provides a better understanding of particle structure, it remains impossible to simultaneously associate this information with the fluorescence profile obtained after staining with fluorescent dyes. We then decided to verify our results using high-resolution brightfield imaging, combining acoustic focusing with a high-speed brightfield camera for simultaneous high-throughput flow cytometry. Importantly, automated image analysis with the Attune Cytometric Software translates event features into distinct morphometric parameters that can be combined with standard fluorescence and scatter parameters. Although optical resolution is a major limitation for brightfield imaging results when studying submicron particles, and that this technology is specifically designed for the analysis of cells and microspheres, we were able to obtain images of MPs and large NPs. However, the very small NPs, approximately 50% of the submicron particles detected by fluorescence, were below the optical resolution limit of the instrumentation, making it impossible to image such small events. Sorting by acoustic focusing-assisted brightfield cytometry can take advantage of other methods based on the simultaneous identification of positive events. In addition, the advantages of flat-top lasers equipped in this instrumentation help to reduce scattered light by approximately 50%, improving the detection of small particles, and also avoiding lateral shift problems in alignment, providing uniform power distribution.
Strikingly, we were able to detect up to one million plastic particles per microliter of blood. The complete blood count in a microliter of peripheral blood corresponds to approximately 5 million cells. Thus, considering that there are one million plastic particles in one microliter of blood, this means that out of every five cells, only one cell could contain a plastic particle. Considering that the abundance of protein molecules in a single cell is counted in the tens of millions, about 42 million to be precise, the plastic quantification we obtained could be a realistic estimate.
Overall, we can conclude that NPs exist in different human tissues and can cross biological barriers, leading to uncertain consequences for human health. Importantly, NPs have been detected, especially in peripheral blood and lymph nodes compared to other fluids used in this study, which gives relevance to the accumulation of plastics at the cellular level. Our results in the bronchoalveolar lavage fluid confirm the possibility that NPs can enter living organisms through inhalation. In fact, the lower accumulation of NPs in mice could be related to the different air quality in facilities equipped with HEPA filters, which allow for the removal of large particles. Age was the main variable influencing NP accumulation in human blood, whereas pathology did not influence it. Newborns had the lowest NP levels compared to all other age groups. The accumulation of NP with age began to increase progressively until it reached a maximum at about 50 years of age. After age 50, accumulation tended to decrease progressively in each decade, suggesting that there may be a relationship between exposure and the accumulation of plastics over time, and of course with increasing plastic production. Interestingly, there were no differences in plastic accumulation with pathology, with the exception of lung cancer patients who had significantly lower blood NP levels. This finding could be attributed to the greater expectoration of these patients and the resulting partial clearance of inhaled particles.
Finally, extrapolation and conclusions based on our data could be highly speculative, even though we studied a large number of human subjects in the Barcelona Metropolitan Area (Spain). To our knowledge, there is no previous literature providing information on plastic accumulation in humans by age, sex, and pathology as we have presented here, and further studies will be necessary to understand the impact of NPs on living organisms as well as their entry, accumulation, and elimination pathways, the long-term effects of plastic exposure, and the development of strategies to mitigate this important environmental threat. In addition, differences between rural and urban areas in terms of sociodemographic, behavioral, and lifestyle factors may vary not only locally, but also worldwide. We are further planning to retrospectively study patterns between rural and urban populations over a ten-year period in two Spanish autonomous communities.