1. Introduction
Induction of immunological tolerance is an interesting therapeutic approach for autoimmune diseases such as rheumatoid arthritis (RA). In relation to this, we designed and developed a novel drug, called Jusvinza, for the control of inflammation associated with RA. The active ingredient of Jusvinza is an altered peptide ligand (APL). The APL closely resembles immunogenic peptides, but it contains one or two substitutions at the specific sites where they interact with T cell receptor or class II HLA molecules. These substitutions have a significant impact on the pathway involved in T cell activation. Among the autoantigens involved in the pathogenesis of RA, we specifically selected the 60 kDa cell stress protein (HSP60) for the design of the APL. This selection was based on the therapeutic potential of HSP60 in the treatment of autoimmune diseases. Our hypothesis was that an APL derived from HSP60 would enhance these therapeutic effects [1]. The HSP60-derived APL was designed using bioinformatics tools. First, a new epitope for T cells was identified in the N-terminal region of the sequence of human HSP60. Then, this epitope was modified in a single amino acid to increase its affinity to the class II HLA molecules associated with RA [2].
Pre-clinical and clinical studies in RA indicated that Jusvinza is safe and reduces inflammation, without causing immunosuppression [3,4]. Recently, the Cuban agency for drug control (CECMED) approved Jusvinza’s medical registration [5]. Jusvinza reduced pro-inflammatory cytokines in several experimental systems [6] as well as in two animal models of RA [2,4]. In addition, it reduced IL-6 levels similarly to tocilizumab in a systemic inflammation model in Zebrafish [7]. Also, Jusvinza treatment reduced antibodies against cyclic citrullinated peptides (anti-CCPs) in RA patients [8]. It is known that NETosis, which is triggered by contact with environmental microorganisms, is the leading source of citrullinated autoantigens in RA [9]. Anti-CCPs, together with rheumatoid factors, precede the onset of disease symptoms and can be used to predict a more severe disease course, indicating their pathogenic role in RA. Neutrophils also play a crucial role in the initiation and progression of RA [10].
In RA disease, both blood and synovial fluid neutrophils have an increased capacity to produce ROS [11,12]. This leads to a microenvironment at joint surfaces with elevated levels of ROS, proteases, and cytotoxic factors, causing damage to underlying tissues [13]. In addition, neutrophil granule proteases not only damage collagen fibers within cartilage but also activate proteins such as matrix metalloproteinases, cytokines, and chemokines [13,14,15]. In addition, ROS production within a joint disrupts the oxidative balance and influences adaptive immune responses in the synovial environment [16]. ROS-induced activation of NF-κB in synovial fibroblasts triggers the production of pro-inflammatory prostaglandins by cyclooxygenase-2 [17]. ROS also affect the local T cell population, contributing to differentiation towards TH17 and TH1 [18]. Neutrophil-derived cytokines and chemokines play a critical role in regulating both innate and adaptive immune responses in RA [15]. Neutrophil apoptosis is also dysregulated in RA, leading to delayed apoptosis within the synovial joints. This contributes to chronic inflammation, immune cell recruitment, and prolonged release of proteolytic enzymes [19,20].
On these bases, Jusvinza was repositioned in our country for the treatment of hyperinflammation that distinguishes patients with COVID-19 and received an Emergency Use Authorization from CECMED [21]. Congruently, in a clinical setting, Jusvinza reduced the inflammation that characterizes COVID-19 patients by decreasing levels of IL-6, TNFα, and IL-10; restoring the physiological serum levels of autoreactive T-lymphocytes; and inducing Treg cells [22]. Activated Treg cells could migrate to inflammation sites and cross-recognize the original epitope of HSP60, which increases its concentration in endothelial tissue, thus inhibiting autoimmune damage to endothelia caused during viral infection [23]. The clinical improvement of COVID-19 patients treated with Jusvinza is also associated with a decrease in several coagulation and inflammation biomarkers [24], including calprotectin. The latter, being the most-abundant cytosolic protein derived from neutrophils, is found at high blood concentrations during inflammatory processes [25,26,27].
Neutrophils are specialized cells of the innate immune system that play a key physiological role in host defense against microorganisms. Currently, three mechanisms have been described by which neutrophils remove pathogens and prevent their dissemination throughout the body. These include phagocytosis, degranulation, and NETosis. Phagocytosis is initiated by the internalization of targeted organisms or particles and enables the effective elimination of pathogens. Neutrophil degranulation involves the sequential release of different granule types containing antimicrobial and cytotoxic proteins [28]. Also, neutrophils can secrete several pro-inflammatory cytokines and molecules associated with inflammation [29]. NETosis is the formation of neutrophil extracellular traps (NETs). NETs, composed of DNA, histones, and proteins from granules and cytoplasm, immobilize and kill microorganisms, thereby maintaining host defense [28]. Nevertheless, dysregulated neutrophil activation contributes to the pathogenesis of inflammatory diseases, such as RA [30] and COVID-19 [31].
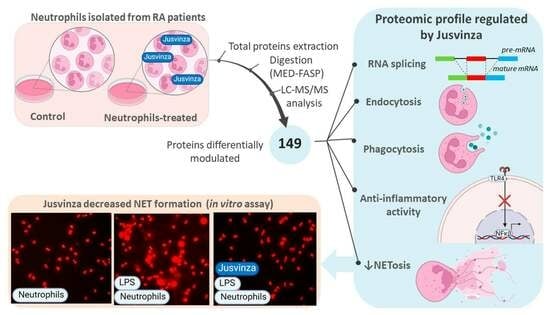
For all these reasons, we determined the total cell proteome regulated by Jusvinza using a primary culture of non-pooled neutrophils which were isolated from four RA patients. Using label-free quantitative proteomics, the molecular perturbations induced by this immunomodulatory peptide were described in neutrophils after 6 h and 18 h of treatment. Importantly, NETosis was validated as a biological process that is counteracted in the presence of Jusvinza. In general, these results provide molecular support to explain the anti-inflammatory effect of Jusvinza and indicate its therapeutic potentialities.
4. Discussion
Jusvinza is based on a classic APL derived from HSP60, which has demonstrated therapeutic effects in RA patients and also in COVID-19 patients with signs of hyperinflammation [8,23,48]. The mechanism of action of Jusvinza is not fully understood. Here, we used label-free quantitative proteomics to identify the Jusvinza-regulated proteome and explore the molecular perturbations promoted by this anti-inflammatory peptide in neutrophils following 6 h and 18 h of treatment. A total of 149 proteins were differentially modulated by Jusvinza treatment, including membrane proteins and low-abundance transcription factors, which are protein classes that usually have low coverage in proteomics datasets. These results demonstrate the usefulness of MED-FASP as a sample preparation method for proteomics analysis [49]. An array of 13 proteins, which are mainly related to RNA splicing or apoptosis-induced DNA fragmentation, were regulated by the peptide at both incubation times (6 h and 18 h). Additionally, the proteomic profile regulated at 6 h in Jusvinza-treated neutrophils was characterized by the presence of proteins related to phagocytosis, endocytosis, lipid metabolism, and immune functions. In comparison with the proteome modulated at 6 h, the effect of Jusvinza seemed to be dispersed at 18 h following treatment (only 54 proteins were differentially modulated). Probably, signal propagation to extracellular events could orchestrate the response to Jusvinza over longer treatment times (18 h and longer times were not covered in this quantitative proteomic experiment).
During the immune system response, neutrophils translocate to sites of infection or acute injury and sequester particulate substrates of microbial or endogenous origin via phagocytosis [50]. Importantly, the hematopoietic lineage cell-specific protein (HCLS1), which mediates neutrophil-directed migration [51,52], was down-regulated at 6 h and 18 h after Jusvinza treatment. In addition, the expression of phosphatase and tensin homolog protein (PTEN), which “prioritizes” neutrophil chemotaxis toward end-target chemoattractants and prevents “distraction” by chemokines during migration [53], decreased at 6 h in response to Jusvinza treatment. PTEN antagonizes the PI3K-AKT signaling pathway; such regulation triggers different effects in the immune system response depending on the cell type [54,55]. Chemotaxis of neutrophils is a cellular process that also depends on cytoskeleton remodeling. Supporting the hypothesis that Jusvinza should hamper neutrophil migration to sites of inflammation, besides down-regulation of HCLS1 and PTEN, the expression of two regulatory proteins of the actin cytoskeleton decreased in peptide-treated cells: Wiskott–Aldrich syndrome protein (WASP) and WAS-interacting protein family member 1 (WIP). On the other hand, proteins related to phagocytosis, which is an essential effector function of the neutrophil-mediated immune response, were down-regulated at 6 h in response to Jusvinza treatment. For instance, the expression of carcinoembryonic antigen-related cell adhesion molecule 4 (CEACAM) decreased eight-fold in response to Jusvinza treatment. CEACAM is a granulocyte orphan receptor that triggers the efficient phagocytosis of attached particles [56]. Based on this result, the influence of Jusvinza on the phagocytic ability of neutrophils should be validated in further experiments. Likewise, proteins related to endocytosis, like clathrin light chain A (CLTA), were down-regulated at 6 h in neutrophils treated with Jusvinza. CLTA is a main structural component of coated pits and vesicles during receptor-mediated endocytosis. Clathrin-mediated endocytosis regulates signal transduction pathways, leading to neutrophil polarization [57], priming of polymorphonuclear neutrophils (PMNs) [58], and priming of respiratory burst activity through ROS-mediated control of granule exocytosis [59]. These events modulate and enhance the neutrophil response at the site of inflammation. Altogether, the down-regulation of proteins related to migration, phagocytosis, and priming of neutrophils constitutes experimental evidence supporting the proposition that Jusvinza could decrease the over-activation of neutrophils, a condition linked to several pathologies, including RA. It would be interesting for future experiments to carry out a proteomic study to evaluate the effect of Jusvinza on monocytes and macrophages. These cells derive from the same myeloid lineage as neutrophils (myelomonocytic stem cells) and also contribute to the pathogenesis of RA [60,61].
Proteins that regulate metabolic functions were identified in this study. Lipid modification and glycerophospholipid metabolism were enriched in the proteomic profile up-regulated at 6 h in response to Jusvinza treatment. Of note, D-beta-hydroxybutyrate dehydrogenase mitochondrial (BDH1) and lysophospholipid acyl transferase 7 (MBOAT7), which are enzymes involved in the synthesis of anti-inflammatory metabolites, were up-regulated in Jusvinza-treated neutrophils. BHD1 catalyzes the interconversion of the two major ketone bodies produced during fatty acid catabolism: acetoacetate and β-hydroxybutyrate (BHB); the latter inhibits NLRP3 inflammasome activation in neutrophils and macrophages and subsequent IL-1 production [62,63]. MBOAT7 negatively regulates free arachidonic acid levels and synthesis of pro-inflammatory leukotriene B4 (LTB4) in neutrophils by catalyzing the conversion of lysophosphatidylinositol into phosphatidylinositol in the phospholipid remodeling pathway (the reacylation step) [64,65]. In addition, perilipin-3 (PLIN3), a protein related to lipid droplet (adiposome) biogenesis and prostaglandin E2 (PGE2) production [66], was down-regulated in response to Jusvinza treatment. PGE2, similar to LTB4, is a lipid mediator produced from arachidonic acid and possesses context-dependent immunoregulatory properties [67]. The molecular functions mediated by BDH1, MBOAT7, and PLIN3 illustrate the close interconnection between the metabolic and immune systems. Therefore, modulation of such proteins in response to Jusvinza treatment suggests that the peptide could drive cellular metabolic reprogramming to exert its immunoregulatory effects. Supporting such a hypothesis, proteins related to fatty acid beta oxidation (ABCD1, ABCD3, ILVBL, and DBI) and aerobic respiration (glycolysis: PKM; the TCA cycle: CS and SUCLG2; mitochondrial respiratory chain complex I: NDUFS7 and NDUFS5) were also modulated in Jusvinza-treated neutrophils. Recent results demonstrate that Jusvinza interacts with apolipoprotein A-I (APOA1) [7,68], the major protein component of high-density lipoprotein (HDL) in plasma. Notably, as demonstrated by network analysis, APOA1 is functionally related to acyl CoA-binding protein (DBI/ACBP), which was down-regulated by Jusvinza. DBI functions as an intracellular carrier of acyl-CoA esters [69] and promotes the expression of genes encoding key enzymes related to glycerolipid, cholesterol, and fatty acid metabolism [70]. DBI as well as APOA1 are annotated in the PPAR signaling pathway according to the KEGG database (KEGG_ID: hsa03320). Besides regulating the cellular response to lipids, the activation of PPARs (peroxisome proliferator-activated receptors) has anti-inflammatory effects on immune cells [71]. Specifically, PPARγ promotes neutrophil apoptosis and clearance during the resolution phase of the inflammatory response [72]. The proteomic profile modulated at 18 h following treatment with Jusvinza includes the protein helicase with zinc finger domain 2 (HELZ2), which functions as a transcriptional activator of nuclear receptors, including PPARα and PPARγ [73]. Importantly, HELZ2, as part of the PPARA coactivator complex, mediates the transcription of APOA1, according to the Reactome database (Reactome ID: R-HSA-1989754). The expression level of HELZ2 increased in Jusvinza-treated neutrophils isolated from three of the four AR patients who were included in the study. The functional association of APOA1, DBI, and HELZ2 with the PPAR signaling pathway, as well as the modulation of proteins related to metabolic pathways that supplies ATP for cellular functions or lipid metabolites with immunoregulatory properties, supports the idea that regulation of metabolism could play an important role in the molecular mechanism of Jusvinza.
As we mentioned before, proteins related to transcription were also modulated in the presence of Jusvinza. Among the transcription factors, the protein IWS1 homolog (IWS1) was down-regulated at 6 h and 18 h, while nuclease-sensitive element-binding protein 1 (YBX1) and the transcriptional regulator ERG (FLI1) were both down-regulated at 18 h after Jusvinza treatment. IWS1, a transcription factor essential for cell proliferation, defines the composition of the RNA polymerase II complex and modulates the production of mature mRNA transcription [74,75]. FLI1 regulates the expression of metalloproteases (MMP-1, MMP-3, and MMP-10) and the pro-inflammatory cytokine IL-10; therefore, during chronic inflammatory conditions, such as AR and atherosclerosis, FLI1 down-regulation could attenuate tissue damage associated with inflammatory responses [76]. The transcription factor YBX1 has also been related to the inflammatory response. In addition to the effect on NF-kB transcriptional activity, in the early phase of inflammation, YBX1 positively regulates CCL5/RANTES chemokine expression [77,78]. YBX1 is secreted by immune cells and was found to be up-regulated during systemic inflammation and vascular damage [79,80]. The decreased expression levels of FLI1 and YBX1 in response to Jusvinza treatment are in agreement with the accumulated evidence demonstrating that the peptide induces an anti-inflammatory response [6,23,24].
In line with these findings, several proteins which regulate NF-κB signaling were modulated in Jusvinza-treated neutrophils. The NF-κB transcription factor is a master regulator of several biological processes, including the inflammatory response. Ligand binding to transmembrane receptors like TNFR, IL1R, and TLR promotes phosphorylation and concurrent activation of the I-kappa-B kinase complex (IKK). This complex promotes ubiquitination and proteosomal degradation of inhibitory IκB proteins, releasing the active NF-κB complex, which is translocated into the nucleus to regulate gene transcription [81]. In response to Jusvinza treatment, IKK interacting protein (IKIP) was up-regulated at 18 h. IKIP inhibits the phosphorylation of IKK and negatively regulates NF-κB activation and pro-inflammatory cytokine production [82]. Similarly, at 6 h following Jusvinza treatment, two proteins that negatively regulate NF-κB signaling were up-regulated: DCC-interacting protein 13-beta (APPL2) and activating signal cointegrator 1 complex subunit 1 (ASCC1). APPL2 is a multifunctional adaptor protein that regulates the LPS/TLR4 signaling pathway by inhibiting nuclear translocation of NF-κB p65 and suppressing inflammatory cytokine secretion [83]. On the other hand, ASCC1 inhibits the expression of NF-κB target genes [84]. A truncated and inactive variant of ASCC1 (p.S78*), which did not reduce the transcriptional activation of NF-κB, has been related to disease severity in RA patients [84]. Furthermore, proteins that activate NF-κB signaling decreased at 6 h and 18 h after Jusvinza treatment. Among these proteins, metadherin (MTDH) and the tumor necrosis factor ligand superfamily member 14 (TNFSF14/LIGHT) were down-regulated at 6 h in response to Jusvinza. MTDH activates the NF-κB pathway by facilitating the degradation of IκB and acting as a transcriptional co-activator of NF-κB p65 [85,86]. LIGHT is considered a pro-inflammatory cytokine that triggers activation of non-canonical NF-κB and STAT3 signaling after binding to receptors (TNFRSF3 or TNFRSF14) [87,88]. Increased levels of LIGHT have been detected in autoimmune and chronic inflammation diseases like Crohn’s disease, coronary disease, RA, and COVID-19 [89,90,91,92]. Specifically, LIGHT promotes RA-FLS (fibroblast-like synoviocyte) proliferation and induces a pro-inflammatory response characterized by increased levels of MCP-1, IL-8, MIP-1α, and ICAM-1 [89]. LIGHT also induces osteoclast differentiation and plays a critical role in the inflammatory joint destruction of RA patients [93]. In the case of COVID-19, LIGHT is related to cytokine release syndrome (CRS), and its expression levels are highly up-regulated in the sera of critical patients who require mechanical ventilation [91].
Other pieces of evidence supporting the impact of Jusvinza on NF-κB signaling were the down-regulation of the HSP90 co-chaperone Cdc37 (CDC37) and leucine-rich repeat flightless-interacting protein 2 (LRRFIP2). Both proteins play a positive role in regulating TLR-mediated NF-κB activation and cytokine production [94,95]. Together with the HSP90 chaperone, CDC37 is a functional component of the IKK complex, mediating its assembly and activation in response to TNF-mediated signaling [96]. The expression level of CDC37 decreased at 6 h and 18 h after Jusvinza treatment, while LRRFIP2 was down-regulated only at later times of incubation (18 h) with the peptide. Besides its role in NF-κB signaling, LRRFIP2 is required for NRL3 inflammasome activation in response to Ca2+ signaling [97]. Therefore, the results suggest that Jusvinza could orchestrate different mechanisms to attenuate the inflammatory process. Relatedly, heat shock factor binding protein 1 (HSBP1), which inhibits the transcriptional activity of heat shock factor 1 (HSF1) [98], was down-regulated in neutrophils in response to Jusvinza. The transcription factor HSF1 is known to inhibit the expression of pro-inflammatory cytokines and mediators (IL-1β, TNF-α, IL-6, HMGB1, and NF-κB) [99,100,101]. In addition, HSF1 functions as a transcriptional activator of the chaperone HSP60/HSPD1, the anti-inflammatory cytokine IL-10, and the zinc finger protein SNAI1, which reduces NLRP3 inflammasome activation [102,103,104]. Interestingly, HMGB1 and HSP60/HSPD1 proteins were down- and up-regulated in Jusvinza-treated neutrophils, respectively. A possible explanation of the differential regulation of HMGB1 and HSP60/HSPD1 might be the decreased levels of HSBP1 and the subsequent activation of the heat shock response (HSR) triggered by HSF1. Of note, HSR-inducing therapies may improve inflammatory profiles in COVID-19 patients with metabolic diseases (obesity and diabetes), which are comorbidities that increase the severity of SARS-CoV-2 infections [105].
Among proteins related to mRNA processing, several components of the spliceosome (SF3B2, SF1, RBM25, and SRSF2) and the exon junction (ACIN1, WIBG, PNN, and UPF3B) complexes were modulated by Jusvinza, including small nuclear ribonucleoprotein G (SNRPG; FC = 8.1), which was the most up-regulated protein at 18 h. Regulation of the splicing machinery could modulate innate immunity. For instance, the inhibition of serine/arginine-rich splicing factor 2 (SRSF2) reduces inflammatory cytokine levels [106], and this protein was down-regulated at 18 h by Jusvinza treatment. Furthermore, nucleolin (NCL), a protein that regulates the ribosome biogenesis pathway by several mechanisms, including the processing of pre-rRNA and ribosome assembly [107], was down-regulated at 6 h and 18 h in Jusvinza-treated neutrophils. In line with modulation of NCL, ribosomal proteins as well as translation initiation and elongation factors were down-regulated in the presence of Jusvinza. Although mainly localized in nucleoli, NCL is also found in cell membranes, promoting the inflammatory response through the internalization of LPS or immunogenic extracellular mitochondria DNA (mtDNA) [108,109]. Increased levels of mtDNA have been detected in synovial fluid samples from RA patients—an event correlated with join inflammation [110]. On the other hand, the expression of plasminogen activator inhibitor 1 RNA-binding protein (SERBP1) was decreased at 18 h in response to Jusvinza treatment. SERBP1 binds to a cyclic nucleotide-responsive sequence located in the 3′-untranslated region of PAI-1 mRNA and regulates PAI-1 abundance by stabilizing or destabilizing PAI-1 mRNA [111]. This dual function of SERBP1 depends on intracellular localization and cellular context [112]. PAI-1, which is up-regulated in COVID-19 patients diagnosed with CRS, promotes endothelial dysfunction and a hypercoagulable state that predisposes to thrombus formation [113,114]. Taking into account the therapeutic effect of Jusvinza for AR and COVID-19 treatments [22,48], validating whether NCL and SERBP1 down-regulation in Jusvinza-treated neutrophils is related to impairment of mtDNA internalization and decreased PAI-1 levels, respectively, could be of interest. Such molecular events could support the anti-inflammatory and anticoagulant effects of Jusvinza [24].
Remarkably, an array of proteins related to nucleosome organization were identified at 6 h and 18 h after Jusvinza treatment. This cluster of proteins included members of the histone family (HIST1H1B, H1Fx, and HIST1H1E) which were down-regulated in the presence of Jusvinza. Histone H1 regulates nucleosome condensation into chromatin fibers. Citrullination of histone H1 mediated by the enzyme peptidylarginine deiminase 4 (PAD4) results in histone H1 displacement from chromatin and global chromatin decondensation; this event is required for extracellular chromatin release during NET formation [115]. As part of the innate immune response, NETs play a beneficial role in pathogen trapping during infection. However, excessive production and/or inappropriate NET removal drive the severe inflammation that characterizes NET-related pathologies like autoimmune diseases and severe COVID-19 [116]. Histones and high-mobility group protein B1 (HMGB1), which was also down-regulated in Jusvinza-treated neutrophils, are NET structural components [116]. HMGB1 is a nuclear chromatin-associated non-histone protein which is also localized in the extracellular environment [117]. Extracellular HMGB1 functions as a danger-associated molecular pattern (DAMP), amplifying the immune response. Through binding to multiple surface receptors (TLR2, TLR4, RAGE, and CXCR4), alarmin HMGB1 leads to downstream NF-κB activation, neutrophil chemotaxis, and NET formation [117,118]. In fact, HMGB1 has been proposed as a potential therapeutic target for severe inflammatory diseases [119,120]. The concomitant down-regulation of NET structural components in the presence of Jusvinza indicates that the peptide could circumvent the inflammatory process mediated by the formation of NETs. Importantly, such a finding was corroborated by in vitro experiments, in which Jusvinza was shown to decrease NET formation in cultures of neutrophils isolated from healthy donors and stimulated with LPS. One limitation of this study is that NETosis was detected by only one method, even though it has been widely reported by several groups [121,122,123]. However, this method was useful in our research, since we could observe how the addition of LPS to neutrophil cultures led them to fall into NETosis. Furthermore, we verified that Jusvinza does not induce NETosis per se and that the addition of Jusvinza with LPS—and even 30 min after adding LPS—avoids the development of NETosis. These results confirm that Jusvinza can be useful for treating diseases associated with an increase in NETosis beyond RA.
Source link
Mabel Hernández-Cedeño www.mdpi.com