1. Introduction
Sepsis, a significant global health challenge, contributes to around 20% of all-cause deaths worldwide, with mortality in intensive care patients being estimated to be between 30% and 40% [
1,
2,
3,
4]. This life-threatening condition arises from a dysregulated host response to infections, including bacterial, viral, or fungal origins. The complexity of sepsis is further emphasized by the varying pathophysiological impacts of different types of infections; for instance, bacterial sepsis typically involves systemic hypercoagulation and fibrinolysis, while severe viral infections like COVID-19 are associated with localized thrombus formation [
5].
The hallmark of sepsis is the disturbance of microcirculation, leading to decreased oxygen delivery, tissue hypoxia, and organ dysfunction. Current resuscitation therapies in septic shock, focusing on fluid therapy and vasopressor support, aim to maintain systemic hemodynamics and counter tissue hypoperfusion [
3,
6]. However, these strategies do not always translate into effective microcirculatory restoration, potentially leading to complications like fluid overload [
7].
The quest for an effective microcirculation assessment has led to the exploration of innovative methods such as remote photoplethysmography (rPPG) and automated capillary refill time (aCRT). These are complemented by techniques like near-infrared spectrometry (NIRS) and hand-held vital microscopes (HCMs), which facilitate bedside microcirculation monitoring. Despite their potential, these methods face challenges in routine clinical application [
8,
9,
10]. Contrast-enhanced ultrasound (CEUS), employing gas microbubbles, has been notably used in patients with COVID-19 to assess lung perfusion anomalies [
8,
11].
Clinically, tissue peripheral perfusion evaluation involves using bedside parameters like the capillary refill time (CRT) and serum lactate levels. The CRT, a dynamic parameter, assesses the skin color return time post-pressure application, with recent trials like ANDROMEDA shock suggesting its role in guiding resuscitation strategies [
12]. Additional indicators such as skin temperature and mottling score provide insights into organ dysfunction severity and patient prognosis [
11,
13]. The Peripheral Perfusion Index (PPI), indicating vascular tone, also offers critical information about microcirculatory changes during sepsis.
However, the manual CRT is subjective, and serum lactate levels lack specificity, underscoring the need for more comprehensive assessment parameters. These include the perfused capillary density, the proportion of perfused capillaries, the microvascular flow index, and the heterogeneity flow index, which are all crucial for understanding the interaction between perfusion and tissue metabolic demands.
This study aims to assess the effects of infusion therapy on microcirculation in patients with sepsis, focusing on the differences between bacterial and viral etiologies. By employing microcirculation assessment techniques such as rPPG and aCRT, we seek to investigate whether bacterial sepsis results in more significant microcirculatory changes compared to COVID-19-associated sepsis.
We hypothesize that bacterial sepsis induces more significant microcirculatory changes than COVID-19-associated sepsis, and that these changes can be detected by rPPG and aCRT techniques.
2. Materials and Methods
2.1. Study Design and Setting
This was a single-center, prospective study conducted at the general intensive care unit (ICU) of Pauls Stradins Clinical University Hospital (PSCUH). The research protocol received approval from the Riga Stradiņš University Ethical Committee (approval number PĒK-22-2/299/2021), adhering to the principles of the Declaration of Helsinki.
2.2. Patient Selection Criteria
This study included 20 patients aged 18 to 70 years who were admitted to the ICU with a diagnosis of severe pneumonia or/and Acute Respiratory Distress Syndrome (ARDS), requiring intensive care. Criteria for inclusion centered on hemodynamic instability, defined as a Mean Arterial Pressure (MAP) < 65 mmHg despite fluid resuscitation and vasopressor use. Fluid resuscitation commenced with crystalloids (normal saline or Ringer’s lactate) at 10 mL/kg/h. Vasopressors were administered according to institutional guidelines, and sedation was achieved using midazolam, fentanyl, and propofol. Exclusion criteria included patients without hemodynamic instability, those outside the specified age range, those who were pregnant, or those with severe cardiac or renal impairments (NYHA class IV heart failure, LVEF < 30%, GFR < 15 mL/min/1.73 m2, or stage 5 CKD).
2.3. Assessment of Fluid Responsiveness and Study Protocol
Fluid responsiveness was evaluated using the Passive Leg Raising Test (PLRT) [
14]. A positive response was defined as an increase in pulse pressure of ≥10% as determined by bedside vital sign monitor [
15]. Patients with a positive PLR test received additional crystalloid infusion at 10 mL/kg/h. The study protocol was organized into four stages: T1—a five-minute baseline assessment in the supine position; T2—PLRT with legs elevated to 45 degrees for 5 min; T3—post-PLRT with legs returned to the baseline position for 5 min; and T4—60 min following fluid infusion.
2.4. Group Stratification
Patients responding positively to PLRT were categorized into two groups:
COVID-19 Group: Ten patients with a positive SARS-CoV-2 PCR test, clinical signs of sepsis, and elevated inflammatory markers (WBC, PCT, and CRP) without secondary bacterial infection.
Bacterial Septic Shock Group: Ten patients with positive blood cultures, clinical signs of sepsis, and increased inflammatory markers.
2.5. Data Collection and Monitoring Techniques
During all stages (T1–T4) of the measurement protocol, macrohemodynamic and microcirculation characterizing parameters were collected from the patients.
2.5.1. Systemic Hemodynamics Monitoring
Continuous evaluation of systemic hemodynamics involved measuring the mean arterial blood pressure (MAP) and recording vasopressor dosages. The MAP was tracked using a Philips Intelevue X3 MX750 bedside monitor(Philips, Eindhoven, The Netherlands) which was directly connected to an arterial blood pressure catheter for real-time monitoring. This allowed for precise adjustments in patient management based on dynamic hemodynamic changes. Vasopressor dosages were also documented from the infusion pumps, offering a comprehensive view of the pharmacological support provided to the patients.
2.5.2. Microcirculation Assessment
Capillary refill time (CRT): CRT was measured through two distinct methods: manual (mCRT) [
16] and automated (aCRT). For the mCRT, a trained investigator applied gentle pressure to the patient’s fingertip until the capillary bed blanched. Upon release, the time taken for the return of normal color was recorded with a chronometer. This traditional approach to assessing peripheral circulation was conducted at heart level to ensure physiological relevance. The aCRT was conducted using a novel custom prototype (Blazar Ltd., Riga, Latvia) with an optoelectronic system designed to provide a more standardized and objective measurement [
17]. The device applied a uniform force (approximately 1 kg, equivalent to 410 mmHg pressure) and used a specific wavelength (525 nm) to detect blood volume changes, enabling the calculation of the total capillary refill time (TST). Both mCRT and aCRT measurements were repeated five times at each time point for accuracy and averaged for analysis. Details on the device operation are provided elsewhere [
17].
2.5.3. Remote Photoplethysmography (rPPG)
Peripheral perfusion was assessed non-invasively using rPPG [
18]. This optical technique uses light reflection to detect changes in blood volume within the microvascular bed. The setup consists of a white LED light source (100 W electric power), an industrial camera (Ximea-xiQ USB-3.0 (XIMEA GmbH in Münster, Germany), ADC 8-12-bits, resolution 648 × 488 pix. with a mounted lens (Edmund Optics, C-mount f = 25 mm (Edmund Optics Ltd., Barrington, IL, USA), and a 540 nm CWL 10 nm FWHM narrow-band filter (Edmund Optics Ltd., Barrington, IL, USA). The dorsal aspect of a patient’s palm was placed at a fixed distance of 30 cm from the light source, and the reflected light signals, indicative of pulsating blood flow, were captured and analyzed. The perfusion index (PI), representing the relative strength of cutaneous blood perfusion, was calculated from the processed single period PPG signal as the ratio between the pulsatile blood flow (PPG signal AC component) to the non-pulsatile blood (PPG signal DC component) in peripheral tissue and recorded at each assessment period [
19].
2.5.4. Temperature Measurements
Dual-mode temperature assessments were conducted. Axillary temperature was recorded using Philips thermometer probe to provide systemic temperature readings. Additionally, regional (cutaneous from the finger) temperature was evaluated using the automated capillary refill time (aCRT) device with an embedded IR thermal sensor MLX90632 (Melexis NV, Tessenderlo, Belgium) at each stage, which aided in assessing localized changes in perfusion.
2.5.5. Serum Lactate Levels
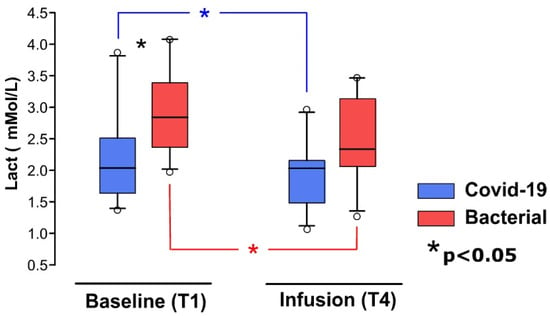
Serum lactate levels, indicative of metabolic status and tissue perfusion, were analyzed at two key stages: the start (T1) and the end (T4) of the study. These analyses were performed using a whole blood gas analyzer (GEM Premier 5000 blood gas testing system, Werfen, Barcelona, Spain) on arterial blood samples. This approach facilitated the assessment of resuscitation efficacy and the detection of potential tissue hypoperfusion.
2.6. Statistical Analysis
Due to the relatively small sample size, nonparametric tests were used in this study. Data were averaged for each stage (T1–T4) and analyzed, comparing the absolute values between the viral and Bacterial groups at each stage. Descriptive statistics were used to present the data as mean ± standard deviation (SD). The Mann–Whitney U test was employed to compare the Bacterial and COVID-19 groups in each phase of the protocol. For within-group comparisons between different protocol stages, the Friedman Repeated Measures Analysis of Variance on Ranks was used, followed by the Student–Newman–Keuls Method for post hoc analysis. Changes in lactate levels within the Bacterial and viral groups at different stages were analyzed using the Wilcoxon Signed-Rank Test. A p-value of <0.005 was considered significant. SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
4. Discussion
Our findings support the hypothesis that remote photoplethysmography (rPPG) and automated capillary refill time (aCRT) techniques are effective in evaluating alterations in peripheral perfusion during fluid resuscitation in patients with septic shock. The perfusion index (PI) measured by rPPG demonstrated higher sensitivity compared to the aCRT. Notably, the PI values significantly differed between the COVID-19 and Bacterial groups, whereas no significant differences were observed in the aCRT parameters between these groups. In our study, we observed changes in microcirculation between the COVID-19 and Bacterial Septic Shock groups, which could be explained by differences in pathophysiological mechanisms. COVID-19 is a unique viral infection with distinct pathophysiological features, particularly in its impact on the coagulatory and inflammatory systems. Our study aimed to compare the microcirculatory effects of bacterial and viral sepsis, with COVID-19 serving as a representative for viral sepsis given its relevance during the pandemic. It is well recognized that COVID-19-induced coagulopathy differs from other viral infections as it is associated with more frequent venous thromboembolism and arterial thrombosis. This contrasts with bacterial sepsis, which typically involves systemic hypercoagulation and suppressed fibrinolysis. COVID-19 involves both direct viral invasion of endothelial cells through ACE2 receptors and indirect damage via cytokine storms, contributing to endothelial dysfunction and microvascular injury [
20,
21]. Despite these distinctions, our study focused on the broader, shared mechanisms of microcirculatory dysfunction in both viral and bacterial sepsis. Sepsis, regardless of its cause, leads to common microcirculatory disturbances, such as endothelial cell damage, glycocalyx degradation, leukocyte adhesion, and microthrombus formation [
22]
Our study results indicate significant differences between groups in terms of noradrenaline consumption, local skin temperature, and serum lactate levels. These differences may be attributed to more pronounced microcirculatory dysfunction in bacterial sepsis compared to COVID-19-associated sepsis.
Previous studies have demonstrated that patients with cold skin on their arms and legs exhibit lower central venous saturation and higher lactate levels [
11,
13]. The research conducted by the Guilherme Martins de Souza group reported similar findings, showing that patient with COVID-19 have a higher Peripheral Perfusion Index (PPI) than patients without COVID-19, as detected by an infrared camera [
23]. The authors suggest that these findings may be related to fewer functional alterations in the microcirculation in COVID-19, indicating that microvascular reactivity dysfunction is not the primary mechanism in the pathophysiology of COVID-19. The research by Matthijs Kox et al. demonstrates that circulating cytokine levels are lower in patients with COVID-19 compared to those with bacterial sepsis [
24]. Although COVID-19-associated sepsis exhibits unique characteristics, particularly in coagulopathy and immune response, it shares critical pathways of microvascular dysfunction with other viral sepsis forms.
In our study, patients with COVID-19 required significantly higher doses of midazolam and fentanyl for sedation, aligning with the findings of Kapp et al. [
25], who reported that patients with COVID-19 with ARDS needed up to three times more sedatives compared to patients without COVID-19. Several factors likely contribute to this increased need, including the use of neuromuscular blocking agents (NMBAs), which often require deeper sedation to prevent awareness under paralysis, as well as high fevers and increased ventilatory drive in patients with COVID-19, exacerbating ventilator dyssynchrony. Additionally, the inflammatory and hypermetabolic state in severe COVID-19 may contribute to increased physical discomfort and ventilatory challenges, further raising sedation and analgesia requirements.
We observed an increase in the perfusion index during the Passive Leg Raising Test in both groups. This suggests that the perfusion index could serve as a sensitive indicator of fluid responsiveness in patients with septic shock, potentially enabling a reduction in the duration of negative fluid balance and the risk of fluid overload. J.L. Vincent, in the Annual Update in Intensive Care and Emergency Medicine [
2], highlighted that the need for fluid administration in response to hypovolemia can be assessed at the bedside by observing a reduced functional capillary density (FCD) and perfused vessel density, which measure diffusive capacity, as well as microvascular flow index or RBC velocity, which indicate the convective capacity of microcirculation [
2]. Furthermore, Duranteau et al. demonstrated that both passive leg raising (PLR) and volume expansion (VE) induced significant improvements in sublingual microcirculation in preload-dependent patients with septic shock [
8].
We did not demonstrate a significant difference between groups when assessing the manual capillary refill time (mCRT). However, when using the automated capillary refill time (aCRT), differences were observed at each PLRT step between the two groups, suggesting that the aCRT is more sensitive than the mCRT. This observation, coupled with the small number of study participants, underscores the potential for the aCRT to provide more reliable measurements. Toll John R, Henricson J, and Anderson CD et al. found that the naked-eye-assessed capillary refill time exhibits poor reproducibility, even among the same observers, and significantly differs from the objectively measured capillary refill time [
26]. Our study demonstrates that the rPPG and aCRT have the potential to provide non-invasive, real-time assessments of microcirculatory function, offering additional insights into a patient’s hemodynamic status. This is particularly relevant in sepsis, where restoring microcirculation is crucial to preventing tissue hypoxia and organ dysfunction. The integration of these novel techniques into routine clinical practice could have several important implications.
One such implication is enhanced monitoring of microcirculation. Currently, most intensive care units (ICUs) rely on systemic hemodynamic parameters such as the Mean Arterial Pressure (MAP) and serum lactate levels to guide resuscitation. However, these markers do not always reflect the state of the microcirculation, which can remain impaired even when the systemic parameters are normalized. By incorporating the rPPG and aCRT into routine monitoring, clinicians would have access to real-time, non-invasive indicators of peripheral perfusion, allowing for more precise guidance of fluid resuscitation and vasopressor therapy.
Another important implication is the early detection of microcirculatory dysfunction. The ability of rPPG to detect subtle changes in the perfusion index (PI) and the objective, automated measurements provided by the aCRT could enable earlier detection of microcirculatory dysfunction, which might not be immediately apparent using traditional methods. This could facilitate earlier interventions aimed at restoring adequate tissue perfusion, potentially improving outcomes in patients with septic shock.
Additionally, these techniques could help reduce the risk of fluid overload. One of the major challenges in managing patients with sepsis is determining the appropriate volume of fluid resuscitation. Over-resuscitation can lead to fluid overload, which is associated with increased morbidity and mortality. By using the rPPG and aCRT to more accurately assess microcirculatory responses to fluid challenges, such as Passive Leg Raising Tests, clinicians could tailor fluid therapy more precisely, reducing the risk of fluid overload while ensuring adequate perfusion.
Finally, improved resuscitation strategies could be developed. In line with the findings of studies like the ANDROMEDA shock trial, which suggested the benefit of perfusion-guided resuscitation strategies, our results indicate that the rPPG and aCRT could be valuable additions to the toolkit used to guide sepsis management. These techniques could complement existing parameters such as the capillary refill time (CRT) and lactate levels, providing a more comprehensive understanding of a patient’s perfusion status.
Limitations of the Study
The findings of this study should be interpreted with caution due to several inherent limitations. However, these limitations are consistent with the preliminary nature of this investigation and do not diminish the study’s contributions to the understanding of microcirculatory dynamics in patients with sepsis.
Firstly, the small sample size (20 patients) is a key limitation, reducing the power to detect more subtle differences between bacterial- and COVID-19-associated sepsis. Small sample sizes are a common limitation in exploratory studies focused on microcirculation, such as those conducted by Dubin et al. (2010) [
27], Edul et al. (2014) [
28], Sadaka et al. (2011) [
29], and Damiani et al. (2015) [
30], which have similarly included 20–22 patients. While our sample size may limit the generalizability of the findings, this study was designed as a pilot investigation to assess the feasibility of using novel techniques—namely remote photoplethysmography (rPPG) and the automated capillary refill time (aCRT)—to monitor microcirculatory changes in a critical care setting. The results provide initial insights into the distinct microcirculatory responses between bacterial and viral etiologies of sepsis. To mitigate the impact of the small sample size, we employed nonparametric statistical tests, which are robust for handling smaller datasets. Future studies with larger cohorts will be necessary to confirm these initial observations and explore more microcirculatory differences.
Secondly, the single-center design of this study limits the external validity and generalizability of the results to other healthcare settings and patient populations. Institutional differences in ICU protocols, patient management, and local demographics could potentially influence the outcomes if this study was conducted in different centers. Nevertheless, the single-center design allowed for the uniform application of inclusion criteria, standardized measurement protocols, and consistent patient care. This consistency reduced potential variability, thereby enhancing the internal validity of the study. Although the results may not be directly transferable to all settings, they provide a controlled proof of concept that can serve as a foundation for future multicenter studies, which will be critical for validating and expanding upon these findings.
The subjectivity inherent in manual capillary refill time (CRT) measurements represents another limitation of this study. The manual CRT is dependent on the observer’s technique, environmental factors such as lighting, and individual interpretation, all of which introduce variability [
26]. To minimize this bias, we employed a standardized protocol, ensured that all manual measurements were performed by a single trained observer, and averaged five measurements per time point to increase reliability. Moreover, we complemented manual CRT with automated CRT (aCRT), an objective measurement method that eliminates observer bias by using consistent pressure and optical sensors to detect the reperfusion upon the blanching. The use of aCRT in conjunction with manual CRT allowed us to compare results and demonstrated that aCRT provided more consistent data across time points and groups. While manual CRT remains widely used in clinical practice, our findings suggest that automated techniques, such as aCRT, offer a more reproducible and reliable alternative, particularly for future research and clinical applications.
Another limitation concerns the clinical significance of the observed microcirculatory changes. While we detected microcirculatory differences between bacterial and viral sepsis using rPPG and aCRT, it remains uncertain whether these changes directly impact clinical outcomes such as survival, organ dysfunction, or recovery trajectories. The relationship between microcirculatory improvement and clinical benefit remains unclear, and, to date, there is a lack of prospective trials specifically targeting microcirculatory endpoints in the resuscitation of patients with sepsis or COVID-19. Our study provides important preliminary data on the feasibility of the non-invasive monitoring of microcirculation, but future research must focus on establishing whether the observed changes translate into improved patient outcomes.
Lastly, as with all observational studies, the potential for residual confounding cannot be entirely excluded. Despite employing a rigorous statistical analysis and controlling for known confounders, there may be unmeasured variables influencing the results. Nonetheless, by using non-invasive, objective tools such as aCRT and rPPG, we minimized the variability introduced by manual methods and enhanced the reliability of our measurements. Future studies should aim to further reduce confounding factors by incorporating multicenter designs and controlling for additional potential confounders.
While this study has several limitations, it provides valuable pilot data on the use of novel, non-invasive techniques for assessing microcirculatory function in sepsis. These findings offer a foundation for larger, multicenter studies that will be essential to confirm and extend the observed differences in microcirculation between bacterial and viral sepsis.