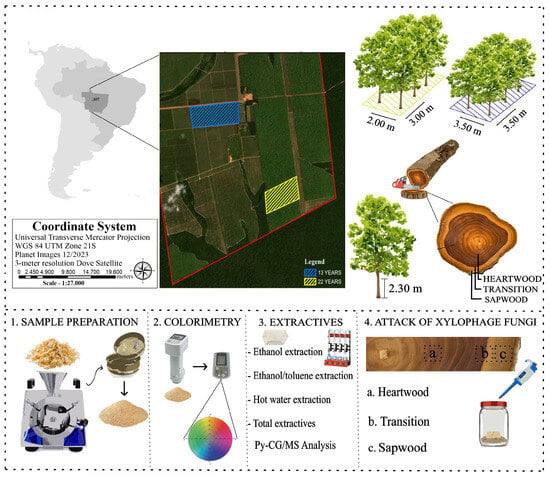
3.1. Colorimetric Parameters
Evaluating the CIEL*a*b* parameters revealed no significant interaction (
p < 0.05) between age and radial position; thus, we evaluated these parameters separately (
Figure 2).
Age had a significant effect (
p < 0.05), except for on lightness. As shown in
Figure 2, the older wood (22yearsold) presented a deeper red tone and a reduced yellow tone, along with lower values of the chroma and hue angle than the younger wood (13years -old) did. In turn, the radial position significantly (
p < 0.05) influenced all colorimetric parameters. The heartwood was darker than the transition zone and sapwood, with a more pronounced red tone, a less intense yellow tone, and lower chroma and hue angle values (
Figure 2). The variation in heartwood color is due to oxidation and polymerization reactions that occur as the wood ages [
25].
Age did not affect the lightness (L*), regardless of radial position (
Figure 2A), with an average value of 59.29. According to the classification of Camargos and Gonçalez [
26], which uses colorimetric coordinates to differentiate light from dark woods, this value is characteristic of light wood (L* > 56). In turn, the radial position influenced lightness, regardless of age (
Figure 2B), with increasing values from the pith to the bark. Teak heartwood is considered dark, regardless of age (L* = 50.39). Given that teak heartwood is naturally dark, regardless of age, this characteristic is viewed as a positive aspect from a commercial standpoint. The reduction in the harvesting age of teak in fast-growing plantations is an important feature, as dark wood is associated with increased density, greater mechanical strength, and natural durability [
27].
The results of the colorimetric studies of teak wood with short rotations were consistent with those of the present study. When evaluating heartwood and sapwood in 5-year-old short-rotation teak plantations, Damayanti et al. [
28] reported a mean lightness value of 60.7. In the study by Arce and Moya [
29], the lightness values ranged from 48 to 59 for 15-year-old clones. A review of the properties of short-rotation teak performed by Moya et al. [
6] presented several studies that, in agreement with the present study, indicated that teak wood from fast-growing plantations is lighter than wood from native trees.
The significant difference in a* between ages (
Figure 2C) suggests that the red hue increases with age, regardless of the radial position. The a* parameter was also different in the radial positions (
Figure 2D), with an increasing value in the pith–bark direction. Damayanti et al. [
28] reported the same radial pattern in 5-year-old trees, with a* values increasing from 9.0 in the sapwood to 12.7 in the heartwood. Similarly, Silva et al. [
12] reported an age-related increase in a* values in teak wood aged 5–20 years, ranging from 3.4 to 5.4.
As in a*, the yellowness (b*) varied independently with age and radial position (
Figure 2E,F). However, b* significantly decreased with the increasing age of the wood and increased in the pith–bark direction. The parameter b* presented higher averages than the parameter a* did, indicating that yellowish coloration is predominant in teak wood. Mesquita et al. [
30] also inferred that there was a predominance of the yellow hue in the formation of the teak wood color, classifying the color of the species as olive brown.
C* decreased with age (
Figure 2G) and was greater in sapwood (
Figure 2H), as was the h° parameter (
Figure 2I,J). Notably, the mean values of h° between 70 and 75° indicate the predominance of yellow in both age groups. According to Silva et al. [
12], teak wood is predominantly yellow, both in younger wood (5years) and older wood (20years), as found by the authors.
3.2. Extractives
As observed for the colorimetric parameters, the extractive contents also showed no significant interaction (
p < 0.05) between age and radial position (
Figure 3). In addition, these factors did not significantly affect the extractive content in hot water (5.07% ± 1.35) (
Figure 4A,B). The radial position also did not significantly affect the ethanol-soluble extractive content (3.93% ± 0.50) (
Figure 4D) or the total extractive content (7.78% ± 0.58) (
Figure 4F). However, for age, regardless of the radial position, the 13-year-old wood showed lower solubility in ethanol (
Figure 4C) and a lower total extractive content (
Figure 4E).
When the solubilities in hot water and ethanol were related to the total extractives, hot water extracted 68% of the total in 13-year-old wood and 61% in 22-year-old wood, whereas ethanol extracted 37% and 63%, respectively. For radial positions, the hot water extraction percentage was 84% in the sapwood, 60% in the transition zone, and 51% in the heartwood; with ethanol, it was 44%, 49%, and 57%, respectively. Although in the present study, no significant difference was found between the radial regions, the absolute values suggest a trend toward increasing total extractives and ethanol content and decreasing extractive water content of the sapwood toward the heartwood. That is, with increasing age and with the formation of heartwood, the compounds become less soluble in water and more soluble in ethanol.Hot water is capable of extracting tannins, gums, sugars, starches, and coloring materials [
31]. In addition, some phenolic substances can be solubilized [
32]. Substances such as starch are found in the axial and radial parenchyma of the outer part of the sapwood, but their concentration decreases dramatically near the transition zone. Moya et al. [
6] attributed this reduction to the histochemical changes that occur due to the death of cells of the radial parenchyma of the sapwood during heartwood formation. On the other hand, because ethanol is a solvent of intermediate polarity, it is able to extract polar compounds, such as lignans and phenolic compounds [
33], in addition to removing relatively nonpolar compounds such as terpenoids and lipids [
34].
Teak wood can have extractive contents between 1.18% and 11.98%, depending on the extraction method used [
32]. Colbu et al. [
35] reported that ethanol, isopropanol, and acetone, together with a mixture of acetone–isopropanol, are the best solvents for the selective extraction of organic compounds with insect fungicide activity from teak wood. Differences in extractive content can also be attributed to factors such as the region of wood and the age of the tree, in addition to environmental and genetic factors [
32].
The mean value of hot water extractives in this study was greater than that reported by Lukmandaru et al. [
36], who reported values ranging from 0.48% to 2.11% in 11-year-old teak wood from Central Java Province. However, it was lower than that reported by Qiu et al. [
10], who reported 9.36% (sapwood) and 10.22% (heartwood) of teak aged 18 years from China. With respect to total extractives, Lukmandaru et al. [
37] reported an average value of 5.59% for the base of 11-year-old trees, whereas Silva et al. [
12] reported values of 7.4 to 9.6 in teak woods aged between 5 and 20 years and planted in Brazil, which is consistent with the values reported in this study.
The older wood was expected to have a higher extractive content (
Figure 4C,E). Rizanti et al. [
38] reported 3.7% total extractives for short-rotation teak (10 years) and 8.0% for long-rotation teak (40 years). Lukmandaru et al. [
36] reported that the total extractive content of the heartwood of a superior clone of teak aged 11 years (4.03~7.31%) was lower than that of the control mature heartwood (13.43%). Previous studies have indicated that heartwood extracts from young trees are less toxic or less abundant than those from mature trees are [
12,
39]. In addition, a quantitative increase in polyphenolic extractives from the pith to the outer heartwood was observed in mature trees [
39]. Notably, after their synthesis, the extractives may continue to undergo chemical transformations, such as oxidation and condensation, with the formation of oligomers and polymeric structures [
40].
3.3. Analytical Pyrolysis (Py-GC/MS)
A total of 119 compounds were identified in the teak wood via Py-GC/MS (
Supplementary Materials). In the 13-year-old wood, the identified area was 98.45% in the sapwood, 97.77% in the transition zone, and 97.49% in the heartwood, with 56, 57, and 58 compounds, respectively. For the 22-year-old wood, the values were 98.15% in the sapwood, 97.99% in the transition zone, and 98.20% in the heartwood, with 61, 61, and 63 compounds, respectively. These results are in agreement with those of previous studies [
41,
42,
43].
Of the total compounds identified, 51 presented an area greater than 1% in at least one of the treatments, and individually, each treatment presented between 24 and 29 compounds with an area greater than 1% (
Table 1). Notably, three quinones, even those with areas less than 1%, were included, totaling the 54 compounds presented in
Table 1.
Acetic acid was the most abundant compound in all the treatments (
Table 1). Its high concentration can be explained by the fact that it is a byproduct of the thermal degradation of cellulose and hemicelluloses [
41].
In addition to acetic acid, for the 13-year-old wood, the main sapwood compounds were as follows: 2-propanone (9.6%), 4-hydroxy-2-methylacetophenone (7.28%), cyclopropyl carbinol (6.32%), 1,2-cyclopentanedione (5.7%), 3′,5′-dimethoxyacetophenone (4.88%), phenol, 2-methoxy-(4.32%), phenol, 2,6′-dimethoxy-(4.12%), and 2-methoxy-5-methylphenol (3.11%). At the transition stage, the main compounds were 2-methoxy-5-methylphenol (7.58%), oxazolidine (5.84%), 4-hydroxy-2-methylacetophenone (5.37%), 3′,5′-dimethoxyacetophenone (3.25%), phenol, 2,6-dimethoxy-4-(2-propenyl)-(3.15%), 4-hydroxy-2-methoxycinnamaldehyde (3.1%), D-limonene (3.06%), and isoeugenol (3.0%).
For the 22-year-old wood, in addition to acetic acid, the following stand out as the main sapwood compounds: 2-propanone (8.47%), 4-hydroxy-2-methylacetophenone (7.27%), cyclopropyl carbinol (5.21%), 1,2-cyclopentanedione (4.43%), d-limonene (4.01%), phenol, 2-methoxy-(3.86%), 3′,5′-dimethoxyacetophenone (3.58%); in the transition zone, the main compounds were ethanone (7.88%), d-limonene (5.59%), 4-hydroxy-2-methoxycinnamaldehyde (5.05%), squalene (4.07%), oxazolidine (3.54%), 2-propanone (3.28%), 3′,5′-dimethoxyacetophenone (3.2%), carbon dioxide (3.12%), and isoeugenol (3.07%); and in the heartwood, the main compounds were ethanone (5.34%), 2-methoxy-5-methylphenol (5.28%), 9,10-anthracenedione, 2-methyl (4.93%), 4-hydroxy-2-methoxycinnamaldehyde (4.23%), oxazolidine (4.1%), 2-propanone (3.51%), 3′,5′-dimethoxyacetophenone (3.37%), cyclopropyl carbinol (3.09%), phenol, and 2,6-dimethoxy-4-(2-propenyl) (3.02%).
In addition to acetic acid, which is a derivative of the thermal degradation of carbohydrates, 2-propanone stood out, especially in sapwood, for both age groups, with values close to 10%. 5-Hydroxymethylfurfural and 3-furanmethanol were found only in the sapwood of both ages; butanedioic acid was found in the transition wood and heartwood of both ages; furanone was found in wood aged 13 years; D-glucopyranose, 2H-pyran-2-one, and 5,6-dihydro-6-pentyl- were found in the 22-year-old wood; 2′,3′-dideoxyribonolactone was found in the 13-year-old heartwood and 22-year-old transition zone; and 1,2-cyclopentanedione was found in higher concentrations in sapwood of both ages.
The compounds 4-hydroxy-2-methylacetophenone, 3′,5′-dimethoxyacetophenone, phenol, 2-methoxy-, phenol, 2,6-dimethoxy-, phenol, 2,6-dimethoxy-4-(2-propenyl)-, 2-methoxy-5-methylphenol, 4-hydroxy-2-methoxycinnamaldehyde, and isoeugenol stood out as lignin degradation products. Generally, products that are derived from lignin are easier to identify, because lignin pyrolysis results in a mixture of phenolic compounds that maintain the aromatic ring, the original methoxyl groups, and portions or the entirety of the propane side chain [
44].
The extractive derivatives 1-hydroxy-4-methylanthraquinone, 1-formylanthraquinone, 2-(hydroxymethyl)anthraquinone, 9,10-anthraquinone, and 9,10-anthracenedione were detected in the transition zone and heartwood of teak wood aged 13 and 22 years. Studies such as those by N’Guessan et al. [
45] and Windeisen et al. [
46] also reported the presence of these compounds in teak wood. The total area of quinones in the transition region is 3.62%, and that in the heartwood is 7.31%, regardless of age. The concentration of quinones in the heartwood of the 13-year-old wood was slightly greater than that in the 22-year-old material (7.54% and 7.08%, respectively), which was unexpected. The slightly higher concentration of quinones in the heartwood of the 13-year-old wood can be attributed both to variations in environmental and growth conditions, which affect the synthesis of these compounds throughout tree development, and to natural variability, especially considering the small sample size in the study.
These compounds confer resistance against attacks by xylophagous organisms; in addition, they have applications in the chemical and food industries [
41,
47,
48]. Vyas et al. [
48], when evaluating the phytochemical potential of
T. grandis, confirmed that these compounds are linked to pharmacological properties such as wound healing; antimicrobial, antioxidant, anti-inflammatory, and cytotoxic effects; and the promotion of capillary growth.
Lapachol, a naphthoquinone, was also identified in this study in the transition and heartwood regions. In the 13-year-old wood, its concentration was 0.87% in the transition wood and 1.13% in the heartwood, whereas in the 22-year-old wood, its concentration was 0.86% in the transition wood and 0.72% in the heartwood. The sapwood of 13- and 22-year-old trees did not contain naphthoquinones. Naphthoquinones are also related to the resistance of teakwood to insects and fungi [
47,
48]. However, Niamké et al. [
47] reported that naphthoquinones are more toxic against fungi than anthraquinones are. Naphthoquinones and their derivatives have antimicrobial, antibacterial, fungicidal, cytotoxic, antiparasitic, insecticidal, and antiulcerogenic effects [
47]. Therefore, even at a low percentage, lapachol offers resistance to attacks by xylophagous organisms in teak wood, especially in the heartwood.
The quinone group is also responsible for wood darkening [
10]. The quinone contents in the heartwood of the 13- and 22-year-old trees were similar (
Table 1), which may explain why the 22-year-old wood was not darker than the 13-year-old wood (
Figure 2A).
Other extractive derivatives detected were D-limonene, which stood out in the transition zone at both ages and in the 22-year-old sapwood, and squalene, which was found in all the treatments, with higher values in the transition zone and heartwood, especially at 22 years. These compounds were also detected in the study by Ismayati et al. [
42], who evaluated teak wood via Py-GC/MS. Squalene has not previously been identified as a factor in natural durability [
39], but it may also serve as a physical or chemical hydrophobic barrier, contributing to the decay resistance of teak [
49].
The sum of quinones and terpenes was 0% (13 years; sapwood), 8.91% (13 years; transition zone), 11.05% (13 years; heartwood), 4.62% (22 years; sapwood), 12.68% (13 years; transition zone), and 12.4% (13 years; heartwood). The value for sapwood was lower than that reported in
Figure 4, indicating that this region of the wood has other extractive components, but not quinones or terpenes. As previously mentioned (
Figure 4), the extractive content was greater in the 22-year-old wood. However, the extractive content here, given the sum of quinones and terpenes, was greater than the total extractive content shown in
Figure 4. Lourenço et al. [
43] reported that extractives were detected by Py-GC/MS, even after the samples had been previously extracted, which highlights the difficulty of completely removing these compounds from the wood matrix.
Other compounds that stood out in the Py-GC/MS data were cyclopropyl carbinol, which was present in more than 5% of the sapwood at both ages; ethanone, which was found only in the transition zone and 22-year-old heartwood at concentrations higher than 5%; and oxazolidine, a nitrogenous heterocyclic compound that was present in all the treatments but at concentrations higher than 3% in the transition zone and heartwood of both ages.
3.4. Durability in Contact with the Trametes versicolor Fungus
Similarly to the other parameters evaluated, there was no significant interaction effect (
p < 0.05) between age and radial position (
Table 2) on the mass loss after the accelerated deterioration test. In turn, age and radial position independently significantly affected (
p < 0.05) mass loss, which was greater for the 13-year-old wood and sapwood (
Table 2).
Table 2 also shows that the sapwood of both the 13- and 22-year-old trees was completely covered by fungal mycelia. In the transition wood, part of the 13-year-old sapwood was fully covered, whereas in the 22-year-old sample, some areas of wood were still visible. Heartwood was the most resistant sample at both ages, although some mycelia were still present.
As expected, sapwood had the greatest mass loss (22.55%), classified as resistant D2017–05 [
50], since the mass loss was between 11% and 24%. Notably, in this region, there were no quinones (
Table 1). In addition, sapwood is lighter in color, with higher b* values and lower a* values (
Figure 2), and tends to have a greater water-soluble extractive content (
Figure 4). Compared with heartwood, sapwood has more primary metabolites, including carbohydrates, lipids, amino acids, and nucleotides [
51]. The storage substances that are present in the sapwood are attractive to fungi that feed on the carbohydrates that are present in the cytoplasm of the cells [
52].
The transition wood lost on average 10.14% of its mass, whereas the heartwood, on average, lost 1.86% of its mass. They were classified as highly resistant (mass loss between 0 and 10%) [
50]. Heartwood presented the highest quinone contents (
Table 1), lower luminosity, higher a* values, and lower b* values (
Figure 2), in addition to a trend toward a greater percentage of ethanol-soluble extractives (
Figure 4). According to Niamké et al. [
32], most extractives from teak heartwood, which are responsible for resistance to deterioration, are synthesized in the transition zone, and the highest levels of extractives are observed in the outer heartwood. With age, the cells lose their physiological function, and there is an increase in extractives that are deposited in heartwood cells, while the sapwood contains unobstructed physiologically active cells that facilitate the entry of xylophagous fungi [
53].
The 22-year-old wood presented a lower mean mass loss (10.30%) than the 13-year-old wood (12.68%) did, with a difference of 2.38%. Previous studies have also shown an increase in the natural resistance of older wood to termites [
39] and fungi [
12]. However, when heartwood, the commercial part of teak wood, was analyzed exclusively, the differences in resistance to deterioration caused by the rotting fungus
Trametes versicolor were not significant, with average losses of 2.79% (13-years-old) and 0.75% (22-years-old). This result is significant, given the increasing use of fast-growing wood from thinning or early harvesting, which suggests that even the heartwood of young trees exhibits high resistance and biological activity. This resistance can likely be attributed to the chemical compounds that are present in the wood, underscoring the potential of younger woods for various applications [
32].
The 22-year-old wood had higher a* and lower b* values (
Figure 2), as well as a higher content of alcohol-soluble extractives (
Figure 4). In contrast, the quinone content in the heartwood was similar across both ages, with slightly higher values in the 13-year-old wood (
Table 1). Notably, the mass loss in the heartwood of 22-year-old trees was more homogeneous, as evidenced by the smaller standard deviation. In contrast, the quinone content in the heartwood was similar across both ages, with slightly higher values in the 13-year-old wood [
39].
Figure 3 shows the relationships between the mass loss and colorimetric parameters, extractives, and quinone contents. Among the variables that are strongly correlated with the mass loss, the colorimetric coordinates, anthraquinones, and total quinones stand out.
With the result of the correlation between lightness and mass loss, the greater the lightness is, the greater the susceptibility of the wood is to the fungus
T. versicolor. In addition, the mass loss was lower with the highest a* value, indicating that the red coloration in the wood increased the natural durability. Furthermore, mass loss was strongly correlated with the b* coordinate (r = 0.820), indicating that yellow wood (sapwood) is more susceptible to decay. The chromatic coordinates of the CIEL*a*b* system can be used to evaluate the natural durability of teak, and the lightness parameter is the best indicator of teak wood’s durability [
32,
54]. This result contradicts the findings of Moya and Berrocal [
54], who did not observe a correlation between the b* coordinate and teak’s resistance to attacks by the fungi T. versicolor and P. sanguineus. They concluded that the significance of the a* and b* color parameters may depend on the fungal species, and therefore, they did not consider these parameters to be good indicators of teak durability.
The moderately positive correlation between the content of soluble extractives in hot water and mass loss (r = 0.677) indicates that wood with a greater amount of these extractives is more susceptible to attacks by xylophagous fungi. This susceptibility can be explained by the chemical composition of the extracts that are soluble in hot water, and the presence of certain sugars and other compounds that are soluble in hot water can provide a favorable environment for the growth of fungi, accelerating their decomposition.
On the other hand, the weak negative correlation between the content of ethanol-soluble extractives (r = −0.349) and the content of total extractives (r = −0.232) indicates that the extractives are associated with greater wood durability, but the determination of the extractive content alone is not sufficient to evaluate the natural durability of teak wood; it is necessary to identify and quantify the specific compounds that are related to its resistance [
12].
In this study, we found a stronger negative correlation between anthraquinones (r = −0.883) and mass loss than between naphthoquinones and mass loss, represented by lapachol (r = −0.818). The total quinone content, the sum of the anthraquinone and naphthoquinone contents, was strongly negatively correlated (r = −0.893) with the mass loss. These results confirm the role of quinones in biological resistance, as demonstrated by other studies [
12,
49]. Our results contradict those of Thulasidas and Bhat [
55], who reported that naphthoquinone is primarily responsible for the resistance of teak to decay by rotting fungi.
Figure 5 shows that earlywood was attacked more often than latewood. This pattern occurs because of the arrangement of anatomical elements along the earlywood at the beginning of growth ring formation and the latewood at the end of growth ring formation.
According to Lepage’s classification [
22], the heartwood of both ages received a score of 2 (index = 70), indicating an evident but moderate attack. The transition region received a score of 3 (index = 40). Finally, the sapwood was the most damaged region, with a score of 4 (index = 0), indicating breakage and a nearly complete loss of strength. According to Bari et al. [
56], the fungus
T. versicolor is able to attack wood via a complex enzymatic system for complete decomposition of the substrate.
Teak wood has distinct growth rings, which are mainly characterized by variations in pore diameter, with semiporous rings [
57,
58]. The pores were large and visible at the beginning of each growth ring, gradually decreasing in size and frequency [
57,
59,
60,
61]. Obstructions were observed in the pores [
57,
62], with notable accumulation of translucent, light-yellow substances in the heartwood region. These obstructions progressively diminished toward the sapwood. The pores were primarily solitary, with occasional twinning and no defined arrangement [
57,
62]. The teak also exhibited radial parenchyma in cross-sections, with axial parenchyma that were arranged in marginal bands [
59,
60,
61] and vasicentric paratracheal parenchyma [
57].
Thus, the greater fungal attack in the earlywood region can be attributed to its greater permeability, resulting from the larger vessel diameters and marginal bands of parenchyma, which create a favorable environment for fungal growth and decomposition. As the fiber wall thickness increases in latewood, the proliferation of xylophagous fungi decreases [
56,
63]. The delignification and simultaneous degradation of lignin and cellulose result in significant damage to the wood structure [
56]. Logically, the attack on the heartwood is less intense due to the obstruction of the pores and higher concentration of toxic extractives.
The proportion of heartwood and the age of teak wood significantly enhance its natural durability. This is attributed to the deposition of secondary compounds, such as phenolic extractives, which are toxic to wood-degrading agents. These compounds contribute to alterations in the color of the wood and an increase in its biological resistance [
14,
62,
64].
Age influences the proportion of heartwood in teak, with older trees exhibiting a higher heartwood proportion [
12]. Additionally, the wood undergoes color changes, becoming darker and more saturated, with yellow hues predominating over red [
12]. The chemical composition and proportion of compounds within the wood are also affected by age, increasing progressively over time [
12].
Another factor influenced by age is the radial variation of teak wood, particularly its color, which tends to reflect the chemical composition of different regions [
28]. Furthermore, the synergistic effects of various extractive compounds significantly contribute to the natural durability of teak [
64].
The heartwood region exhibits a higher deposition of extractives due to processes such as heartwood formation, cambial maturation, programmed cell death, and the filling of vessels with secondary compounds [
65]. In contrast, the sapwood, which constitutes the physiologically active portion of the wood, has lower biological resistance and a lighter coloration [
65]. The anatomy of teak wood is influenced by radial variation, with larger vessels generally being observed in the sapwood region, gradually decreasing in size towards the pith [
66]. Additionally, the proportion of ray parenchyma cells increases from the pith towards the outer region [
66], contributing to radial variations in the wood. These structural differences directly affect the proportion and distribution of compounds, which in turn influence the utility and commercialization of teak wood [
12,
64].