1. Introduction
Modern decision-making in the treatment of spinal metastases considers neurological compromise, radiosensitivity, spinal stability and overall patient performance. Some patients need surgical intervention for stabilization or tumor decompression [
1]. Most often, stabilizing the spine can be achieved via a pedicle screw–rod construct. However, under certain circumstances, vertebral body replacement (VBR) is necessary. On a regular basis, titanium implants are used for spinal instrumentation. Such implants have the potential to induce imaging artifacts, which may subsequently compromise the accuracy of postoperative imaging and radiation treatment planning [
2,
3]. Therefore, minimum metallic instrumentation (MMI) has been propagated as a new standard of care for spinal tumors by various leaders in the field. With modern implants made of polyetheretherketone (PEEK) or carbon fiber-reinforced PEEK (CRF-PEEK), metal hardware components can be reduced significantly [
4]. The value of CRF-PEEK dorsal instrumentation has been the subject of extensive study in recent years, including investigations into its implications for adjuvant radiation treatment planning [
4,
5,
6,
7,
8,
9]. Only one case series on CRF-PEEK vertebral body replacement has examined the clinical and radiological outcomes of patients with thoracolumbar tumors [
10]. To date, there has been no published comparative report on the impact of minimal metallic vertebral body replacement (VBR) on radiotherapy planning for spinal metastatic disease. The objective of this study is to analyze the implications of using different VBR materials for photon radiation therapy planning in a proof-of-concept setting.
4. Discussion
Following surgery for spinal metastatic disease, the radiation oncologist starts planning a consolidating radiation therapy. This is usually realized as fractionated photon beam irradiation via intensity modified radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT). Both planning approaches are characterized by conformal dose distributions, enabling the optimal sparing of normal tissue and organs at risk with complex beam setups and dynamic collimation via multi-leaf collimators. Our case study compares radiation treatment planning after VBR with different materials. Using Monte Carlo calculations, our work shows that radiation plan quality was nearly identical in PEEK and titanium VBR systems. The standardized ICRU plan quality parameters from the ICRU Report 83 especially did not differ significantly. The same is true for the heterogeneity index and conformity index indicating similar quality in terms of heterogeneous dose distribution and target volume coverage, respectively. These results are in line with a proton/photon therapy planning study on different dorsal fixation systems composed of CRF-PEEK or titanium. Interestingly, the authors demonstrated that CRF-PEEK dorsal fixation in superior in the planning and execution of proton radiation therapy [
18]. However, proton therapy is only available in highly specialized centers and is not defined as a standard of care for adjuvant radiation therapy in spinal metastatic disease.
Only a few studies have been published on non-titanium VBR. Shen et al. report on a series on an integrated custom composite polyetheretherketone/carbon fiber VBR in the treatment of bone tumors of the spine [
19]. Recently, a larger series on CRF-PEEK VBR was published by Schwendner et al., reporting on a promising clinical evaluation of CRF-PEEK for vertebral body replacement in patients with thoracic and lumbar spine tumors. Still, no clinical studies have tried to objectify possible differences in treatment planning for the adjuvant photon radiation of spinal metastatic disease, a common clinical situation. Only one experimental cadaveric study reported that PEEK VBR resulted in a significantly more uniform distribution of therapeutic radiation compared with titanium [
6]. There is a paucity of evidence that PEEK is inferior to titanium in spinal instrumentation in terms of mechanical properties. However, long-term arthrodesis rates following CFR-PEEK dorsal instrumentation have not yet been published in the literature [
7]. Studies on other spinal implants like interbody cages and cervical anterior plating systems suggest high fusion rates [
20,
21].
Monte Carlo algorithms are highly advantageous for radiotherapy planning involving metal implants due to their unparalleled ability to simulate the intricate interactions of radiation with heterogeneous materials. These algorithms account for scattering, attenuation and secondary particle production, providing accurate dose calculations even in areas where simpler methods like pencil beam algorithms fall short [
22]. A prerequisite for accurate photon radiotherapy is a planned CT including densitometric information. This is followed by the delineation of target volume and organs at risk, a process called contouring. Metal-related image artifacts directly find their way into radiation planning by affecting manual and semi-automatic segmentation [
23]. While modern planning CT setups implement AI-based artifact suppression as well as on-board imaging during RT, this is not standard yet [
24,
25,
26]. Additionally, the semi- or fully automated, as well as artificial intelligence-driven, segmentation of organs at risk is becoming more prevalent in modern radiotherapy planning. In this context, metal artifacts can cause errors in auto-segmentation, disrupting the workflow [
23]. Therefore, PEEK will probably facilitate the transformation to emerging new artificial intelligence-driven auto-segmentations. Visually, the utilization of PEEK demonstrated an advantage in contouring paraspinal target volumes as evidenced by enhanced radiolucency and minimal artifact formation. These aspects are of particular significance to radiation oncologists. MMI can be an option for treating cases of metastases extending within and beyond the spine due to its ability to provide improved visualization, which is facilitated by a reduction in artifacts. This expands the utility of MMI in clinical practice, particularly in the context of high-precision irradiations. These techniques, also known as SBRT (Stereotactic Body Radiation Therapy), offer the advantages of delivering high-dose radiation precisely to spinal tumors while minimizing therapeutic margins and target volumes, resulting in effective tumor control with fewer treatment sessions and reduced side effects. Optimal treatment planning imaging is needed for SBRT. Here, particular parts of the vertebra are precisely segmented to only irradiate relevant volumes, as opposed to the whole vertebra in standard radiotherapy [
27]. As the use of SBRT is steadily increasing, improved visualization with the precise delineation of target and surrounding structures is crucial, thus indicating the need for artifact-free implants [
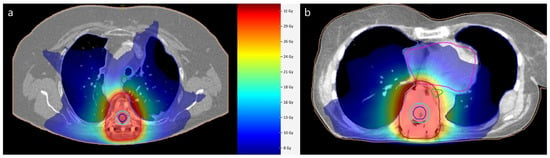
28]. Furthermore, the determination of the dose to the spinal cord as an organ at risk is important in spinal radiation oncology. Modern concepts also incorporate the integrated protection of the spinal cord to minimize neurotoxicity. The improved visualization after MMI enables a reasonable delineation of the spinal canal, as seen in
Figure 1. Despite methodological efforts to reduce metal artifacts, MRI image quality is superior to that in MMI [
4]. Especially for long-term follow-ups of benign lesions such as spinal dumbbell schwannomas in close proximity to implants, the full advantages of MMI should be exploited [
29].
Although derived from only a small cohort of patients, our findings may have implications for treatment plan decisions within interdisciplinary tumor boards. There is a need for individualized interdisciplinary decision-making in the treatment of spinal metastatic disease, e.g., if there is a need for high-precision irradiations adjacent to implants. This approach underscores the importance of tailoring treatment plans to the specific needs of each patient, considering factors such as tumor location, the extent of disease and available resources. By optimizing treatment strategies in this manner, healthcare providers can achieve more cost-effective solutions without compromising patient care or outcomes. The decision to use one hardware over another can be achieved only if radiation oncologists and spine surgeons understand that with thoracic paraspinal metastatic masses, an MMI approach should be chosen.
The most immanent limitations of this case series are the small sample size, inter-individual heterogeneity and retrospective nature. Correlations between plan quality and clinical data, such as cancer type, would be of great interest and need to be addressed in future studies. It is important to note that this proof-of-concept study compares measures of treatment planning. Only comparative cadaveric analyses, such as in the pilot study performed by Jackson et al. in 2017, can contribute to demonstrating actual dose distributions [
6]. Still, these experimental settings do not represent the true biology of spinal metastatic disease with varying extents of spinal and extraspinal tumor burden. The translation of the results to the treatment of lesions in other parts of the spine is desirable but poses challenges. In the cervical spine, VBR is often followed by anterior plating, which may involve titanium implants. This additionally complicates analysis and may hinder direct comparisons of thoracic spine metastatic disease, as in this study, with the rest of the spine. Despite this, it is essential to recognize that the thoracic spine is most frequently affected by osseous metastases, underlining the relevance of this study’s findings in a clinical context. The heterogeneous group of etiologies within the study population presents another limitation. The diversity of underlying causes may introduce confounding variables, making it difficult to draw meaningful comparisons in terms of oncological outcomes.