1. Introduction
1.1. The Background and Significance of Lithium Iron Phosphate Batteries
1.1.1. The Background of Lithium Iron Phosphate Batteries
1.1.2. The Significance of Lithium Iron Phosphate Batteries
Lithium iron phosphate, as a core material in lithium-ion batteries, has provided a strong foundation for the efficient use and widespread adoption of renewable energy due to its excellent safety performance, energy storage capacity, and environmentally friendly properties. Advancements in the research and development of this material, along with technological breakthroughs can not only reduce the dependence on external energy sources but also enhance energy independence and resilience. This is achieved by accelerating the integration of lithium iron phosphate as the core of energy storage systems, thereby improving the flexibility and reliability of power supply, which is crucial for the stable operation of the economy and society.
1.2. The Objectives of This Review
2. The Development of Lithium Iron Phosphate Battery Materials
2.1. Synthesis Method for Lithium Iron Phosphate Cathode Materials
2.1.1. The High-Temperature Solid-Phase Method
In the ball milling method, conditions such as the ratio of powder to milling balls, milling time, temperature, speed, and the amount of solvent all have significant effects on the reaction outcome. Below is a detailed analysis of the impact of these conditions:
- I.
The Ratio of Powder to Milling Balls (Ball Milling Ratio)
- II.
Milling Time
- III.
Temperature
Therefore, large ball mills require water cooling, and the mill must be cooled to room temperature before opening.
- IV.
Speed
- V.
Amount of Solvent
Solvent plays a role in reducing friction, cooling, and lubricating during the ball milling process. However, the amount of solvent needs to be controlled within a certain range.
2.1.2. Hydrothermal/Solvothermal Syntheses
2.1.3. Microwave Reaction Method
2.1.4. Carbon Reduction
In industry, the high-temperature solid-state method is currently the most mature and widely used synthesis method for lithium iron phosphate. On the one hand, Guoxuan High-tech Co., Ltd. (Hefei, China) and Hunan Yuneng New Energy Battery Materials Co., Ltd. (Xiangtan, China)are the representative enterprises producing lithium iron phosphate through the high-temperature solid-state method. On the other hand, Hubei Wanrun New Energy Technology Co., Ltd. (Shiyan, China) and Jiangsu Lopal Technology Co., Ltd. (Nanjing, China) produce their lithium iron phosphate materials via the carbon reduction method.
Please note that the advantages, disadvantages, and production volumes listed in the table are based on general overviews and may vary depending on specific process parameters, equipment conditions, raw material quality, and other factors. In practical applications, the selection of the preparation method should be comprehensively considered based on specific needs. Additionally, as technology continues to progress and innovate, the advantages, disadvantages, and production volumes of various preparation methods may also change.
2.2. Doping and Surface Modification Strategies
2.2.1. Doping
2.2.2. Coating
Carbon Coating
Metals and Their Oxides
Conductive Polymer Coatings
2.3. Nanostructure and Morphology Control
3. High Load Electrode Manufacturing Technology
3.1. Binder and Conductive Additive Optimization
3.2. Porous and Nanostructured Electrode Design
4. Electrolytes and Additives
4.1. Electrolytes
As a medium for ion transport, electrolyte formulation is directly related to electrochemical performance and battery stability. Generally, the electrolyte consists of a solvent system, lithium salt, and various functional additives to further optimize performance. The electrolyte solvent systems of lithium iron phosphate batteries mainly include mixtures such as ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC). Each solvent contributes uniquely to the overall performance of the electrolyte due to its physicochemical properties. For example, EC facilitates efficient ionic conduction due to its high dielectric constant, whereas PC offers high solubility for lithium salts and has suitable melting and boiling points.
4.2. Additives
4.3. Solid Electrolytes for Lithium Iron Phosphate Batteries
The use of solid-state electrolytes in lithium iron phosphate batteries is important for the development of current battery technologies. All-solid-state batteries, which use inorganic solid-state compounds as electrolyte materials, offer high energy density, nonflammability, and other characteristics that significantly improve both safety and energy storage capabilities.
Lithium iron phosphate is currently a high-quality commercialized cathode material, and its combination with sulfide solid-state electrolytes, known for their excellent lithium-ion conductivity (>10 mS/cm), is considered a promising technological route. However, in practical applications, sulfide electrolyte materials have a narrow electrochemical stabilization window, with a large gap between their oxidation limit (~2.2 V) and the Fe2+/Fe3+ redox potential of the lithium iron phosphate cathode (~3.45 V). This discrepancy makes sulfide materials more susceptible to chemical reactions with lithium iron phosphate, resulting in intrinsic electrochemical incompatibility between the sulfide–lithium iron phosphate interfacial system, which negatively impacts the performance of the battery.
At present, what has been mass production on board or will soon be on board the solid-state battery is actually mostly semi-solid-state batteries, compared to the full solid state battery that converts pure solid state electrolyte into a solid–liquid hybrid electrolyte. Although pure solid-state batteries have many advantages, they are subject to high manufacturing costs and are currently unable to achieve mass production. However, with the continuous progress of technology, solid-state batteries are expected to be more widely used in the future.
5. Battery Design and Manufacturing Technologies
Cathode: made of LiFePO4 material with an olivine structure, connected to the battery’s positive terminal via aluminum foil;
Anode: composed of carbon (graphite), connected to the battery’s negative terminal via copper foil;
Separator: Made of polymer and located between the cathode and anode. It separates the cathode and anode while allowing lithium ions to pass through but not electrons;
Electrolyte: Filled inside the battery. It is used for the conduction of lithium ions;
Case: the battery is enclosed in a metal case.
Charging Process: Some lithium ions are extracted from LiFePO4, pass through the electrolyte to the anode, and are then embedded in the carbon material of the anode. At the same time, electrons are released from the cathode and travel to the anode through an external circuit to maintain the balance of the chemical reaction. During this process, LiFePO4 gradually loses lithium ions to form FePO4.
Discharging Process: Lithium ions are detached from the anode, pass through the electrolyte to the cathode, and at the same time, electrons are released from the anode and travel to the cathode through an external circuit to provide energy to the outside world. During this process, lithium ions are embedded into FePO4 to form LiFePO4.
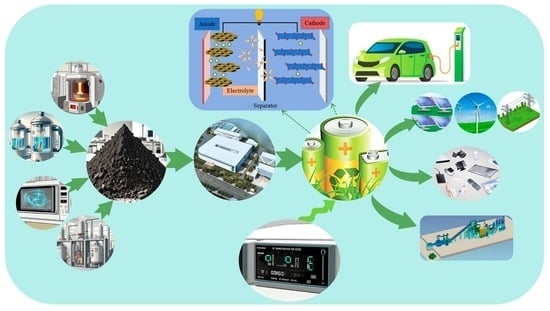
Due to its excellent performance, the LFP battery has a wide range of applications in multiple fields.
LFP batteries have a wide range of applications in the field of new energy vehicles, especially in buses and special vehicles. They serve as powerful batteries and provide power to support new energy vehicles. LFP batteries are also commonly used in energy storage systems, such as solar energy storage and wind energy storage. They can store electrical energy and release it when needed to provide stable power support to the grid. In addition, LFP batteries are also used in electric bicycles, electric tools, uninterrupted power supplies (UPSs), and other fields.
5.1. Battery Assembly and Encapsulation Methods
5.2. Diaphragm Materials
5.3. Current Collectors and Battery Architecture Optimization
In addition, the optimized design of the battery architecture is equally critical to improving key performance indicators, such as energy density, power output, and cycle stability. For example, multilayer electrode stacking technology can significantly increase the load of active material per unit volume, thereby effectively improving the energy density of the battery while maintaining the same volume. Moreover, fine adjustments of the internal space layout of the battery can optimize the flow path of the electrolyte, ensuring full infiltration into the electrode material, reducing ion transmission obstructions, lowering internal resistance, and improving charge/discharge efficiency.
6. Performance Enhancements and Security Features
6.1. High-Magnification Performance and Cyclic Stability
6.2. Thermal Management and Safety Mechanisms
In contrast, liquid cooling utilizes a coolant circulation system to leverage the high heat transfer capacity of liquids. This enables rapid and uniform heat transfer across the battery pack, making it suitable for high-power output and high-energy-density battery systems. Liquid cooling significantly improves thermal management efficiency, ensuring stable operation of the battery under extreme conditions.
6.3. Overcharge and Overdischarge Protection
7. The Applications of Lithium Iron Phosphate Batteries
7.1. Electric and Hybrid Vehicles
7.2. Renewable Energy Storage and Integration
7.3. Portable Electronic Products and Consumer Equipment
In today’s society, portable electronic products such as smartphones, tablets, and laptops, which rely on advanced battery technologies like lithium iron phosphate, have become lighter and more efficient, significantly impacting people’s lifestyles. These devices, with their compact size and light weight, easily accommodate the diverse needs of communication, entertainment, and work. Smartphones are particularly prominent for integrating communication, information access, and entertainment, thereby making connectivity ubiquitous. Tablets enhance experiences such as reading, watching movies, and light office work with their wide displays.
7.4. Grid-Scale Energy Storage Systems
It also overcomes the limitations of pumped storage (due to geographical constraints) and compressed air storage (due to efficiency losses), providing diverse options for grid energy storage. In application, lithium iron phosphate energy storage systems are not limited to peak frequency regulation but have also become key to promoting large-scale grid-connected renewable energy (such as solar energy and wind energy). By suppressing the volatility of renewable energy generation, the phenomenon of “abandoned wind and light” can be significantly reduced, promoting the efficient use of clean energy. In the event of grid failures or emergencies, lithium iron phosphate energy storage can quickly provide backup power to maintain the stability of power supply to key facilities and users. In addition, its ability to participate in power market transactions creates a new profit model and growth point for grid operators and investors.
8. Environmental and Sustainability Considerations
8.1. Life Cycle Analysis and Environmental Impact Assessment
8.2. Resource Availability and Recovery Strategies
The availability of lithium iron phosphate resources depends to some extent on the reserves of lithium resources. With the sharp increase in demand for lithium-ion batteries, the demand for lithium resources has also risen significantly. However, lithium resources are unevenly distributed, and their mining and supply are constrained by a variety of factors, such as the difficulty of mining, the quality of the resources, and the market price. Although the current supply of lithium resources can still meet the demand, in the long run, it is necessary to continue to pay attention to the exploration, mining, and rational utilization of lithium resources in order to ensure its sustainable supply.
Recycling strategies are critical in order to improve the utilization of lithium iron phosphate resources and reduce environmental impact. Below are some common lithium iron phosphate recycling strategies and methods:
(2) Wet recycling: Using wet metallurgy, the valuable metal elements in the cathode materials of waste lithium iron phosphate batteries are leached out. Elements such as lithium, iron, and phosphorus enter the solution in the form of ions and can be recovered separately after the removal and purification of lithium and iron . For example, lithium is generally recovered in the form of lithium carbonate and lithium phosphate, and iron is generally recovered in the form of iron phosphate and iron hydroxide. Commonly used leaching agents are inorganic acids (e.g., sulfuric acid, nitric acid, hydrochloric acid, etc.), but organic acid leaching can also be used, and the oxidizing agent usually uses hydrogen peroxide;
In the recycling process, attention needs to be paid to environmental protection and safety issues to avoid secondary pollution. At the same time, the efficiency and economy of recycling technology should be improved to promote the development of the lithium iron phosphate recycling industry. In addition, strengthening the publicity and education of consumers, improving the awareness of battery recycling, and improving the battery recycling system will also help to improve the recycling rate of lithium iron phosphate resources.
8.3. Circular Economy Approach for Lithium Iron Phosphate Batteries
8.3.1. The Recycling of Lithium Iron Phosphate Batteries
8.3.2. Battery Reuse and Life Extension
Recovered lithium iron phosphate batteries can be reused. Using advanced technology and techniques, the batteries are disassembled and separated, and valuable materials such as lithium, iron and phosphorus are extracted from them. These materials, after reprocessing, can be reused to produce new batteries or other products, upon the recycling of resources. At the same time, extending the life of the battery is also an important strategy of the circular economy. By optimizing the battery design and manufacturing process, the performance and stability of the battery can be improved and the loss can be reduced. In addition, the development of intelligent battery management systems to precisely control the charging and discharging process and avoid overcharging and overdischarging helps to extend the service life of batteries.
8.3.3. Resource Sharing and Sustainable Development
9. Challenges and Future Prospects
9.1. Remaining Technical Challenges and Constraints
Lithium iron phosphate is currently facing a number of technical challenges and limitations. In terms of energy density, due to the limitations of the material’s performance, it has less room for improvement and is close to the upper limit, which makes it slightly insufficient under the high requirements of electric vehicles in terms of range and intelligent functions. Although there are research attempts to advance lithium iron phosphate batteries through material process innovation, such as the exploration of lithium manganese iron phosphate, the overall improvement is still limited. At the same time, in terms of recycling, the stability of lithium iron phosphate material brings difficulty in recycling, and there are many problems in the traditional recycling method, such as complex process, high energy consumption, low product purity, high recycling cost, and low income. Moreover, traditional recycling often focuses on anode materials and insufficiently takes into account other components, which is easily causes new environmental problems, and new recycling technologies and methods are still under research and development.
In addition, lithium iron phosphate has some other problems. Its low-temperature performance is not good; in a low-temperature environment, the battery performance will drop significantly, affecting the range and the usefulness of the battery. In addition, the cost and price are also affected by a variety of factors, such as frequent and drastic fluctuations in the price of raw material lithium carbonate, which will increase the cost of power battery companies, which may be transmitted to the downstream consumers and adversely affect the development of new energy vehicle industry. However, researchers and enterprises in related fields have been actively working hard, expecting to overcome these challenges through technological innovation and improvement and expanding the performance and application scope of lithium iron phosphate.
9.2. Emerging Trends and Research Directions
In addition, improving low-temperature performance and extending cycle life are also key research directions. In terms of low-temperature performance, it is important to develop new electrolytes and additives, such as using electrolytes with lower freezing points and better ionic conductivity, as well as additives that can improve electrode surface performance. For longer cycle life, optimizing the composition of the electrolyte and improving the battery management system is essential to reduce capacity degradation and increase internal resistance during cycling by precisely controlling the charging and discharging strategy. There are also battery system integration optimizations, such as CTP (Cell to Pack) technology and blade batteries, which can significantly improve integration efficiency and bring system energy density close to that of ternary batteries. Additionally, by optimizing the structure of the battery pack and thermal management system, it can also improve the safety and reliability of the battery system, laying the foundation for the application of lithium iron phosphate battery in a wider range of fields.
9.3. Market Prospects and Commercialization Prospects
The market outlook and commercialization prospect of lithium iron phosphate is optimistic. In terms of market size, China is an important producer and consumer of lithium iron phosphate batteries in the world. The global market capacity reached RMB 138,654 million in 2023, and China’s market capacity is also considerable, and it is expected that the global market size will grow to RMB 125,963.4 million by 2029 at a CAGR of 44.72%. The current market situation is highly concentrated and dominated by leading enterprises such as Ningde Times and BYD, but the competition is getting more and more intense, and new entrants are facing greater challenges due to technical and financial thresholds.
In terms of market prospects, lithium iron phosphate has obvious advantages. In the electric vehicle market, its safety and high thermal stability are suitable for electric buses, commercial vehicles, etc. In the electric tools and portable equipment market, long cycle life and low self-discharge rate make it a reliable choice. In the energy storage system, its market safety, stability, and low cost make it a competitive option. In terms of developmental trend, the industry is moving towards technological innovation and upgrading, diversified applications, and green environmental protection. The development of the new energy vehicle industry has brought about a huge demand market, and the diversification of application scenarios for energy storage batteries has also highlighted their advantages, while technological innovation has improved their range and competitiveness. However, the industry is facing challenges such as energy density improvement and raw material price fluctuations, but the overall outlook is good, and the potential is huge. Enterprises and the government need to work together to promote its healthy development.
10. Conclusions
As an important cathode material for lithium-ion batteries, lithium iron phosphate has the advantages of high theoretical capacity, chemical stability, and safety, which is significant in energy security and industrial upgrading strategy. Its anode material synthesis methods include high-temperature solid-phase method, hydrothermal method, microwave reaction method, carbon reduction method, etc. The electrochemical performance can be enhanced by doping, surface modification, nanostructure control, and morphology control. Highly loaded electrode fabrication requires the optimization of binders, conductive additives, and the design of porous and nanostructured electrodes. Reasonable selection of electrolytes and additives can optimize the battery performance; currently, solid-state electrolyte is the development direction, but there are interface compatibility problems. Battery design and manufacturing should focus on the optimization of assembly, packaging, diaphragm, collector, and battery architecture. Lithium iron phosphate battery has a high performance rate and cycle stability, and the thermal management and safety mechanisms include a variety of cooling technologies and overcharge and overdischarge protection. It is widely used in electric vehicles, renewable energy storage, portable electronics, and grid-scale energy storage systems. In terms of environment sustainability, life cycle analysis is required to adopt appropriate recycling strategies and circular economic methods. Currently, challenges such as energy density enhancement, recycling, and low-temperature performance are the prevalent concern. The future should be dedicated to increasing energy density, improving low-temperature performance, and developing new preparation technologies. The market outlook is optimistic, but enterprises and the government need to work together to cope with competition and challenges to realize a sustainable development strategy.
Source link
Tao Chen www.mdpi.com