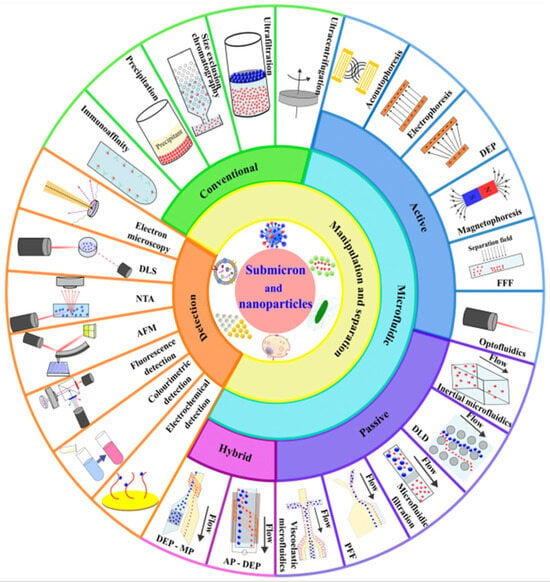
2.4.1. Direct Selective Magnetic Separation
A multitude of methods are available for the separation of complex chemical mixtures. These methods usually exploit the intrinsic physical and chemical properties of the substance, such as solubility, evaporation and fusion temperature, binding affinity, and so on. On occasion, however, it would be advantageous to impart new chemical or physical characteristics to the molecule that requires isolation, therefore enhancing the efficiency of the separation or isolation process and simultaneously lowering costs and processing time. Magnetism is a fascinating characteristic possibly attributed to atoms, ions, or molecules. While many materials possess a certain level of magnetism, the level of magnetism directly affects the susceptibility of a material to controlled magnetic fields (below 1 Tesla), achievable by electromagnets or permanent magnets.
The use of magnetic NPs is widely employed also because of the plethora of methods for their preparation. Excellent overview on the preparation of magnetic NPs is given in recent reviews [
88,
89,
90].
As the suspended fluid flows through a microchannel, magnetized micro-collectors trap the magnetic particles, allowing for their microfluidic separation. Because of their extremely high Brownian motion, nanoparticle separation could be challenging. One limitation of nanoparticles is their low capture efficiency, which limits their use in a bio-analysis. The efficacy of nanoparticle capture can be greatly improved when magnetic nanoparticles undergo field-induced aggregation, which increases the magnetic attractive force as a function of aggregate volume [
91,
92].
An inherent advantage of including magnetically responsive substances into a liquid mixture, such as water, is their ability to be selectively extracted and isolated from the other constituents of the solution using a magnet. This eliminates the necessity of introducing commonly employed solvents or reagents, particularly during the recovery phase [
93]. Nevertheless, extremely complex molecules pose a challenge in terms of transferring magnetic properties to compounds that require isolation. The proposed strategy to tackle this problem focuses on the utilization of functionalized magnetic particles (MPs). Magnetic particles (MPs) are specifically engineered to engage with and attach to a certain category of chemical on their surface, therefore generating a sufficient magnetic moment to extract the molecule from the water or liquid combination.
It is fundamental to understand that each pollutant possesses a distinct binder, sometimes referred to as an affinity ligand. In blends containing several target pollutants, a combination of functionalized MPs can be employed. Typical magnetic particles (MPs) consist of iron oxide (Fe
3O
4) and have been employed by many organizations for the separation and recycling of nanoparticles, e.g., [
94,
95]. The choice of Fe
3O
4 was based on its limited environmental impact and its low chemical toxicity towards humans, plants, and other species [
95]. The functionalization of MPs occurs either after synthesis with specific ligands utilizing advanced methods [
96] or, alternatively, after acquisition.
Wang et al. [
96] reported that magnetic nanomaterial demulsifiers exhibit excellent demulsification performance, are recyclable, and encompass a broad spectrum of possible applications. Furthermore, they are efficacious and ecologically sustainable. They could potentially provide the solution to the issues related to emulsified oily effluents in various sectors and the demulsification of emulsions generated by the petroleum and lubricant industries. Upon the completion of the demulsification process, the magnetic nanoparticles can be recycled by the application of an external magnetic field.
Another approach to enhance the demulsification process is to functionalize the magnetic particles, thus increasing the specificity of their surface (
Figure 7).
As an example, forming a sponge-like structure of a poly(ionic liquid) matrix (PIL matrix) on the top layer of the Fe
3O
4/SiO
2 support, a magnetically recyclable catalyst with a high magnetization value (14.3 emu/g) and open active sites to reactants, was carried out. The Fe
3O
4@SiO
2@PIL catalyst, which used a high loading of the active component, demonstrated excellent catalytic performance in the esterification of oleic acid (92.1%) for biodiesel generation. This performance can be attributed to the functionalizing layer structure of poly(sulfonated vinyl imidazolium ionic liquid). Furthermore, the catalyst exhibited robust stability and reusability, in addition to its convenient recovery in the presence of an external magnetic field [
96].
Lamdab et al. [
97] utilized magnetic MnFe
2O
4 nanoparticles synthesized by co-precipitation at pH values of 9.0, 9.5, 10.0, and 10.5 to effectively eliminate colors from wastewater, with rhodamine B serving as a representative contaminant. Following the adsorption of the dissolved contaminant, the magnetic nanoparticles can be separated using an ordinary magnet. Similarly, the magnetic nanoparticles can be retrieved after the demulsification of an emulsion (
Figure 8).
2.4.2. Combination of Microfluidics with Magnetic Fields
Microfluidic devices employ magnetic field-based separation techniques extensively in biomedical applications for disease diagnoses, including cancer detection, medication delivery, and hyperthermia therapy. Bahadorimehr et al. [
98] exploited magnetic force to separate magnetic nanoparticles from the liquid in which they were immersed. A comprehensive microfluidic system for the separation of functionalized magnetic beads has two primary elements: a fluidic section and a magnetic section. The utilized nanoparticles feature dimensions of 90 nm, 200 nm, and 750 nm. This technique entails the precise introduction of magnetic nanoparticles into a microchannel. Underlying the channel, a permanent magnet is strategically placed to provide a magnetic field that exerts an influence on the nanoparticles. Consequently, the magnetic nanoparticles become oriented in a manner that aligns them above the permanent magnet. The determined parameters include the trajectory, velocity, and capture position of magnetic nanoparticles.
In their work, Orlandi et al. [
91] examined how field-induced aggregation affects the effectiveness of capturing magnetic nanoparticles (about 80 nm in diameter) in a microfluidic channel equipped with a nickel micropillar array. The experimental configuration consisted of subjecting this array to an external uniform magnetic field with an intensity measuring between 6 and 10 kA/m. The flow rates considered in the trials ranged from 0.3 to 30 L per minute. Agnihotry et al. [
99] showed that microfluidics offers a cost-effective method for generating concentrated magnetic nanoparticles from dispersed sources. The synthesized nanofluids were introduced into microchannels while they were exposed to a magnetic field. Empirical studies have shown that the rate of input flow plays a crucial role in controlling the concentration of nanofluid.