1. Introduction
Inonotus hispidus (Bull.) P. Karst. belongs to Basidiomycota, Agaricomycetes, Hymenochaetales, Hymenochaetaceae,
Inonotus. It primarily parasitizes broad-leaved tree such as
Morus alba L.,
Ulmus macrocarpa Hance,
Fraxinus mandshurica Rupr.,
Ziziphus jujuba Mill., and
Malus pumila Mill.
I. hispidus parasitizing on
M. alba is mainly distributed in the Shandong, Hebei, and Xinjiang regions of China. The fungus parasitizing on
F. mandshurica is primarily found in Jilin, Heilongjiang, Liaoning, and other parts of northeastern and northwestern China [
1,
2]. Researchers have employed non-targeted metabolomics and whole-genome sequencing to reveal the differences in metabolites and the genetic basis of
I. hispidus parasitizing on
M. alba and
F. mandshurica [
3,
4]. However, there is still a lack of comprehensive understanding regarding the root microbial diversity and secondary metabolites of these two host plants, which hinders the medicinal development of
I. hispidus on different tree species. We speculate that this may be related to the root microbial diversity and secondary metabolites of the host plants
M. alba and
F. mandshurica. Therefore, it is essential to identify the types and richness of endophytic bacteria, endophytic fungi, and differential metabolites in the roots of these different hosts.
In the natural environment, various microorganisms inhabit different parts of plants, both on their surfaces and interiors. These microbial communities are collectively referred to as the plant microbiome [
5]. Plants provide numerous niches for the growth and reproduction of microorganisms, including bacteria, fungi, protozoa, nematodes, and viruses [
6]. Over the past decade, research on the plant microbiome has focused on the rhizosphere, phyllosphere, and plant endophytes, especially rhizosphere microorganisms or root endophytes. These microorganisms can influence plant metabolism through known and unknown biosynthetic pathways, promote the reconstruction of plant metabolism, and help plants resist various environmental stresses [
7,
8]. Due to long-term co-evolution, plant endophytes and their hosts have established a mutually beneficial symbiotic relationship, allowing endophytes to metabolize substances originally produced only by plants, resulting in the production of the same or similar compounds [
9]. It is well known that the structure, function, and assembly of plant root microbial communities have garnered significant attention from researchers, becoming a hot spot and frontier in microbiology research [
10]. Root microorganisms affect plant growth and health by promoting nutrient uptake and utilization, enhancing the host immune system, and aiding in adaptation to abiotic stresses [
11]. Because root endophytes are a natural biological resource, they hold unique and significant application value, with potential uses in medicine, food, industry, and agriculture. This study focuses on the root endophytes of two host plants of
I. hispidus,
M. alba and
F. mandshurica growing
I. hispidus, exploring the diversity of microbial communities and the relationship among both trees, their endophytes and
I. hispidus. Furthermore, it investigates how these host plants promote the growth of
I. hispidus, providing a theoretical basis for understanding their relationship in the wild environment.
Metabolomics is an emerging field that emerges genomics, transcriptomics, proteomics, and it is an important component of systems biology. Metabolomics is Metabolomics is used to study the endogenous metabolites of organisms or cells during specific physiological periods, and it is an important component of systems biology. As a critical biotechnology method, widely targeted metabolomics technology has been extensively applied in plant science research due to its high precision, high throughput, and broad coverage [
12]. Plant root metabolites are categorized into primary and secondary metabolites. Primary metabolites are the main energy substances involved in plant growth, metabolism, and life-sustaining activities, including carbohydrates, lipids, amino acids, nucleic acids, and other macromolecular compounds. Secondary metabolites, produced by plant secondary metabolic pathways. They include phenolic acids, terpenes, flavonoids, steroids, and alkaloids [
13]. Additionally, plant metabolites act as “messengers” for signal exchange, playing a crucial role in plant-microorganism interactions [
14]. Root metabolites serve as important carbon sources for soil microorganisms, providing a natural medium for screening soil microbial flora and regulating rhizosphere microorganisms. There are few studies on the system network related to plant-microorganism interaction mediated by root metabolites. Therefore, based on the plant-microorganism interaction model, one focus is to systematically elucidate the differences in root metabolites between the host plants
M. alba and
F. mandshurica to understand the relationship between the host and
I. hispidus from a metabolomics perspective.
In summary, understanding the differences in root microbial composition and metabolites between M. alba and F. mandshurica, the host plants of I. hispidus, is essential for elucidating the correlation between I. hispidus and its hosts. Additionally, it is important to investigate the correlation between these differences and the variation in effective components of I. hispidus on different hosts. This study uses high-throughput sequencing of the 16S rRNA V3 + V4 region and ITS2 region to analyze the community composition, alpha diversity, beta diversity, and functional prediction of endophytic bacteria and fungi in the roots of M. alba and F. mandshurica. Additionally, UPLC-MS/MS combined with widely targeted metabolomics was used to identify and analyze the chemical components of the root systems of M. alba and F. mandshurica. Multivariate statistical analysis methods such as PCA and OPLS-DA were employed to quantitatively analyze differential metabolites and enrich the metabolic pathways of these metabolites. Through combined microbiome−metabolome analysis, this study clarifies key differential bacteria and metabolites in the different hosts of I. hispidus. This understanding of changes in the endophytic microbial community structure of the two hosts may explain differences in the fruiting body size and metabolites of I. hispidus, providing a scientific basis and reference for expanding the development and utilization of I. hispidus resources.
2. Materials and Methods
2.1. Root Samples Source and Pretreatment
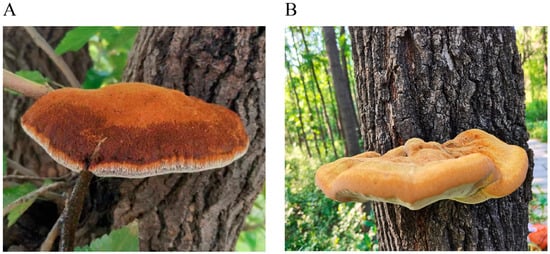
In October 2023, the root endophytes of
M. alba and
F. mandshurica, the host plants of
I. hispidus, were selected for study. The roots of
M. alba were collected from the ancient
M. alba forest in Xiajin County, Dezhou City, Shandong Province (36°59′ N, 115°11′ E) (
Figure 1A), and the roots of
F. mandshurica were collected from Jingyuetan Forest Park, Changchun City, Jilin Province (43°79′ N, 125°46′ E) (
Figure 1B). Six samples were randomly taken from each group, consisting of mature roots from healthy and robust plants free of diseases and pests. The total length of the roots was approximately 5 cm. In total, 12 samples were collected, stored on dry ice at −80 °C, and immediately transported to the laboratory, where surface disinfection was completed within 24 h.
First, the surface of each plant root sample was washed with running water, and small lateral roots were removed. The root soil particles were chemically disinfected with 95% sodium hypochlorite for 2 min, and bacteria were physically removed by vigorous shaking with sterile glass beads in sterile water. Afterward, the root tissue was cut open with a surgical blade to release the endophytes. The root tissue was then shaken with sterile glass beads in 9% saline at 30 °C for 4 h to isolate the endophytes. The endophytes were filtered with a 5 μm filter membrane, centrifuged at 15,000 rpm at 4 °C to collect the precipitate, and stored in liquid nitrogen. The samples of endophytic bacteria were designated as MARTV and FMRTV, respectively, while the samples of endophytic fungi were designated as MAITS and FMITS.
2.2. Metabarcoding Survey
The total DNA of the samples was extracted using the DNeasy PowerSoil Kit (QI AGEN, version 1.9.1, Hilden, Germany), following the manufacturer’s instructions. The concentration and purity of the DNA were determined and diluted to 10 ng/μL. The bacterial barcode amplification region was the V3 + V4 region of 16S rDNA, with primer sequences 341F: CCTAYGGGRBGCASCAG and 806R: GGACTACNNGGGTATCTAAT. The fungal barcode amplification region was the ITS2 region of ITS, with primer sequences ITS3_2024F: GCATCGATGAAGAACGCAGC and ITS4_2409R: TCCTCCGCTTATTGATATGC. The diluted genomic DNA was used as a template for PCR amplification with specific primers containing barcode sequences. For PCR amplification, 15 µL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswic, MA, USA), 0.2 μM primers, and 10 ng of genomic DNA template were mixed. The PCR protocol included an initial denaturation at 98 °C for 1 min, followed by 30 cycles at 98 °C for 10 s, 50 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min. PCR products were detected by 2% agarose gel electrophoresis. Qualified PCR products were purified using magnetic beads, quantified by enzyme labeling, and mixed in equal amounts based on PCR product concentration. After thorough mixing, the PCR products were detected again by 2% agarose gel electrophoresis, and the target bands were recovered. Library construction was performed using the NEBNext Ultra II DNA Library Prep Kit for Illumina Hiseq2500, and high-throughput sequencing was carried out using the PE250 sequencing platform at Novogene Bioinformatics Technology Co., Ltd., China (Beijing, China).
The raw sequencing data were spliced using FLASH (version 1.2.11). Samples were distinguished based on barcode, and low-quality sequences were preliminarily removed. Quality control was conducted using QIIME2, filtering out sequences with an average mass of less than 30, a length of less than 200 bp, and any ambiguous bases (N). The ASV feature table (Amplicon Sequence Variant feature table) was generated after noise reduction and chimera removal. Repeated sampling and removal of low-frequency ASVs were performed to normalize the data with the minimum sample size as the standard.
Species classification datasets for the Unite database (ITS2 region) and the SILVA database (16S V3 + V4 region) were constructed using a classifier based on the Naïve Bayes algorithm, which was used to annotate the ASV characteristic sequences. Mothur was used for alpha diversity analysis. Beta diversity analysis employed GuniFrac (version 1.0), vegan (vesion 2.5.3), and other software packages, considering both Weighted Unifrac and Unweighted Unifrac algorithms to calculate distances between samples and determine the degree of difference between them. ANOSIM analysis was used to evaluate the differences in the composition of endophytic bacterial and fungal communities between groups. Finally, PICRUSt2 (version 2.3.0) and FUNGuild (Fungi Functional Guild) were used to predict the functions of endophytic bacteria and fungi, respectively.
2.3. Widely Targeted Metabolome Analysis
The roots of M. alba and F. mandshurica, consistent with the endophyte samples, were selected and recorded as MARTW and FMTRW, respectively. The root samples of M. alba and F. mandshurica were dehydrated. The dried roots were then ground into powder using a grinding machine at a power of 30 Hz for 1.5 min. Fifty milligrams of lyophilized powder was dissolved in 1.2 mL of 70% methanol extract, vortexed for 30 s, and repeated six times over 30 min. The samples were refrigerated at 4 °C overnight. The sample was then centrifuged at 12,000× g for 3 min, and the supernatant was collected. The sample was filtered with a microporous membrane (0.22 μm pore size) and stored in an injection vial for UPLC-MS/MS analysis.
For chromatographic analysis, mobile phase A was ultrapure water with 0.1% formic acid, and mobile phase B was acetonitrile with 0.1% formic acid. The elution gradient was as follows: 0–9 min, 5–95% min B; 9–10 min, 95% B; 10–11.1 min, 5–95% B; 11.1–14 min, 5% B. The flow rate was 0.35 mL·min−1, the column temperature was maintained at 40 °C, and the injection volume was 2 μL.
The mass spectrometry conditions were as follows: ESI source operating parameters included a source temperature of 550 °C, an ion spray voltage of 5500 V (positive ion mode)/−4500 V (negative ion mode), ion source gas I (GSI), gas II (GSII), and curtain gas (CUR) set at 50, 60, and 25 psi, respectively. The collision-induced ionization parameter was set to high. The QQQ scan used MRM mode, with the collision gas (nitrogen) set to medium. Further optimization of de-clustering potential (DP) and collision energy (CE) was performed for each MRM ion pair. Specific sets of MRM ion pairs were monitored for the metabolites eluted in each period.
Using the self-built novoDB database (novogene database) on the UPLC-MS/MS detection platform of Beijing Novogene Bioinformatics Technology Co., Ltd., China, the software Analyst 1.6.3 was used for qualitative and quantitative analysis of mass spectrometry, including base peak detection, peak filtering, and peak alignment to obtain the corresponding peak area and relative content. R (version 2.15.3) software was used for principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) on the overall metabolite and metabolite accumulation patterns of samples in each group. Substances with RSD > 30% in QC samples were filtered out in data quality control. Differential metabolites in each group were screened based on the significance threshold (p < 0.05), variable importance in projection (VIP > 1), and fold change ≥ 2 or fold change ≤ 0.5 (FC). The differential metabolites were then annotated and analyzed using the KEGG database.
2.4. Correlation Analysis
The top 20 differential metabolites of M. alba and F. mandshurica roots were selected for correlation analysis with the relative abundance of differential genera at the corresponding 16S/ITS genus level. The Pearson statistical method was used to calculate the correlation coefficient between the relative abundance of each differential genus and the quantitative values of different differential metabolites at the genus level, and the chord diagram was drawn using the R language ggalluvial.
4. Discussion
There are about 700 kinds of medicinal fungi in China, and edible and medicinal fungi has become the sixth largest crop in the agricultural field after grains, cotton, oil, fruits, and vegetables. Among these fungi,
I. hispidus, is commonly known as traditional Chinese medicine “Sanghuang”. At present, functional foods made from
I. hispidus, such as “
Sanghuang tea”, are popular with people. It has been reported that 1353 metabolites with varying relative abundances have been identified in
I. hispidus growing on five different tree species. The contents of principal components and trace elements were different. For example,
I. hispidus growing on
M. alba trees was mainly enriched in polysaccharides, phenolic metabolites, and trace elements such as Ca, Na, Mg, Fe, and Mn; and the contents of puerarin, quercetin, and apigenin were significantly enriched in
I. hispidus growing on
F. mandshurica trees, which had the highest crude fat content of 7.67% [
3]. However, the community structure of endophytic bacteria and endophytic fungi, and the mechanism of secondary metabolites in the roots of
M. alba and
F. mandshurica are still unclear. In this study, we used the roots of two host plants of
I. hispidus,
M. alba and
F. mandshurica, as research objects. We detected the endophyte diversity and differential metabolites, in order to provide a scientific basis for further development and utilization of wild medicinal resources of
I. hispidus.
For a long time, with the rapid development of high-throughput sequencing technology, plant microbiology research has made significant progress. Numerous studies have shown that in the process of long-term co-evolution with host plants, a close and complex mutually beneficial symbiotic relationship has formed between endophytes and host plants [
15]. Endophytic bacteria in host plants provide nutrition and physical protection to ensure their growth and reproduction, while obtaining a stable living environment from the host. Some endophytic fungi have also been found to produce the same or similar active substances as the host, and some are potential sources of new natural products. Endophytes have the ability to produce structurally diverse secondary metabolites during co-evolution with the host, showing antibacterial, anti-tumor, and nerve cell protection activities. This study analyzed the differences between
M. alba roots grown in a warm temperate monsoon climate and
F. mandshurica roots grown in a temperate continental monsoon climate from the perspective of endophytic bacteria diversity. High-throughput sequencing of the 16S V3 + V4 region and ITS region was completed, and the community structure, composition, abundance, diversity, and function prediction of endophytic bacteria and endophytic fungi in roots were compared to elucidate the function of the core microflora of
I. hispidus growing on different hosts. The results showed a large number of microorganisms in the roots of
M. alba and
F. mandshurica, with most being unknown microorganisms.
The statistical analysis of endophytic OTUs revealed that the diversity of bacterial OTUs was much higher than that of fungal OTUs. However, it still truly reflected the distribution of endophytic diversity between two samples, suggesting that the endophytic bacteria and fungi community may be more sensitive and more vulnerable to environmental impact than the endophytic bacterial community. In this experiment, the most abundant phylum, cyanobacteria, was obtained by 16S rRNA amplification and sequencing. However, it is worth considering that the most abundant phylum of cyanobacteria was not exposed to light, which may be because the residue of host plants affected the main factors of bacterial community composition, but did not affect the accuracy of 16S rRNA amplicon sequencing. Ascomycota and Basidiomycota are the dominant fungal groups in the roots of
M. alba and
F. mandshurica. Studies have shown that Ascomycota and Basidiomycota can decompose difficult-to-degrade complex organic matter such as lignin and cellulose in the soil and can survive saprophytically, parasitically, and symbiotically [
16,
17]. Ascomycota can withstand more environmental pressures and utilize more resources, resulting in a wider range of nutritional strategies and enhancing their advantages in harsh environments [
18]. Basidiomycota can utilize more difficult-to-decompose carbon and adapt to low-nutrient environments [
19]. Ascomycota dominated in different seasons and growth stages, which may be related to changes in litter quality during decomposition, indicating that Ascomycota is a key driver of nutrient cycling and energy flow in the roots of
M. alba and
F. mandshurica. Researchers studying the diversity of fungi in the rhizosphere soil of
Tricholoma matsutake found that the main fungi in the rhizosphere soil belonged to Ascomycota, also present in
Russula and
Craterellus [
20,
21]. Subsequent alpha and beta diversity analyses demonstrated that the bacterial diversity distribution of
I. hispidus on different hosts was similar, and the low abundance of endophytic bacteria and fungi may be an important part of the ecosystem composition of
I. hispidus. Beta diversity results indicated differences in the structure of endophytic bacteria and fungi, and the abundance of dominant or specific bacteria between the two groups, which is consistent with the finding that rhizosphere microbial community structure is affected by soil types [
22].
PICRUSt2 and FunGuild are software tools for predicting the ecological functions of bacteria and fungi, respectively. They predict the functional potential of bacterial and fungal communities based on marker gene sequencing profiles, offering low-cost and high-reliability alternatives to metagenomic research [
23,
24,
25]. Studies have shown that changes in soil fungal communities can increase ecosystem stability and are closely related to the growth and development of host plants. This study found that endophytic bacteria in the roots of
M. alba and
F. mandshurica mainly included four fungal groups: saprotroph, symbiotroph, pathotroph-symbiotroph, pathotroph-saprotroph-symbiotroph. Generally, saprophytic nutritional fungi, mostly classified as Ascomycota, decompose organic matter such as plant residues. Mycorrhizal fungi, important representatives of symbiotic nutrition, have a symbiotic relationship with plants, aiding nutrient absorption while extracting lipids and carbohydrates from host plant roots [
26,
27]. This study found that the ectomycorrhizal group in
M. alba roots was significantly higher than that in
F. mandshurica roots, highlighting their role in promoting
M. alba growth and development. However, further exploration is needed to understand the physiological activities of ectomycorrhizal fungi on their hosts. Many root fungal functions remain unidentified, indicating the complexity of fungal community functions requires further study.
Plant metabolites are rich and complex, covering a variety of species, and single detection methods cannot fully reveal plant metabolic information. Widely targeted metabolomics covers qualitative and quantitative analysis of most metabolites in plants from primary to secondary metabolism, providing a comprehensive view of plant metabolic systems. It has been widely used to compare the differences in metabolic components of different plant varieties. In this study, widely targeted metabolomics detection technology was used to analyze the differences in types and contents of metabolites in the roots of
M. alba and
F. mandshurica, the host plants of
I. hispidus. A total of 1231 metabolites in 20 categories were identified, and 562 major differential metabolites in 19 categories were screened, including 270 significantly up-regulated and 292 significantly down-regulated metabolites. These included flavonoids, amino acids and their derivatives, sugars and their derivatives, lipids, terpenes, organic acids and their derivatives, phenolic acids, and other substances. The main metabolites of
M. alba roots were Isohyperoside, Sanggenone H, Aspartic acid di-O-glucoside, Tricetin O-hexoside, Quercetin 5-O-hexoside, and others, while the main metabolites of
F. mandshurica roots included Forsythoside A, Isoacteoside, Forsythoside I, Fraxin, 3-Hydroxyoctadecanoic Acid, and others. These abundant metabolites in
M. alba and
F. mandshurica may affect the metabolites of
I. hispidus growing on them, which requires further verification. The number of detected metabolites far exceeds traditional detection methods, indicating that UPLC-MS/MS metabolomics detection technology is more sensitive and systematic, providing a powerful tool for identifying active substances in medicinal plants and mining new drug source molecules. But it is worth noting that, the previous extraction was the fruiting body of
I. hispidus [
3]. This paper is concerned with the extraction of the root system of the host plant of
I. hispidus, which leads to the previous characteristic metabolites not being detected.
KEGG is a powerful tool for in vivo metabolic analysis and metabolic network research [
28,
29]. Using KEGG Pathway as a unit, a hypergeometric test was applied to find pathways enriched in differential metabolites compared with all identified metabolite backgrounds. Pathway enrichment can determine the most important biochemical metabolic pathways and signal transduction pathways involved in differential metabolites. The results of KEGG enrichment in this paper showed that the differential metabolites in the roots of
M. alba and
F. mandshurica, the two host plants of
I. hispidus, mainly included 46 metabolic pathways such as fructose and mannose metabolism, diterpenoid biosynthesis, and caffeine metabolism. There were 11 metabolites in total that were significantly different in fructose and mannose metabolism. Among them, L-Sorbose and alpha-D-Glucose were more abundant in
M. alba roots. Nine metabolites, including D-Sorbitol, D-Mannitol, and D-Mannose-6-phosphate, were significantly enriched the roots of
F. mandshurica. These results indicated that the endophytic bacteria in the roots of
M. alba and
F. mandshurica promoted the growth of
I. hispidus by producing plant hormones or promoting plant nutrient absorption, especially the acquisition of nitrogen, carbon, and phosphorus. Secondly, this may be related to the activity of enzymes or the expression of genes in the related metabolic pathways between the two host plants of
I. hispidus. However, there are few studies on the changes in secondary metabolites in the roots of
M. alba and
F. mandshurica. Therefore, the specific change mechanism of root secondary metabolites between different hosts needs to be further explored.
With the vigorous development of multi-omics technology, it is possible to shift from fine decomposition research to the overall research of the system. The method of studying microbial flora is 16S rDNA and ITS amplicon sequencing. Usually, one or several variation regions are selected, and universal primers are designed using conserved regions for PCR amplification, followed by sequencing and identification of the hypervariable regions. 16S rDNA and ITS amplicon sequencing technology have become important means to study the composition and structure of microbial communities in environmental samples. Metabolomics can measure the metabolic changes in the host ecosystem at a certain time point. Therefore, it is necessary to study the phenotypic changes that may be caused by changes in the structure of the host microbial community, and to analyze the correlation between the metabolic group and the microorganism. The data of different omics complement and verify each other, revealing the differences and commonalities between the two host plants of I. hispidus. The results of this research paper provide a basis for further study on the internal relationship between I. hispidus and its different hosts.