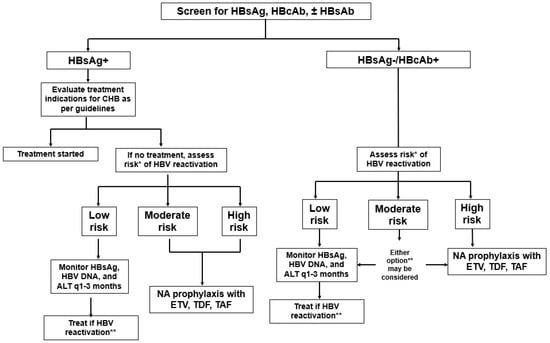
As with non-transplant settings, patients with CHB and latent HBV who are solid organ transplant recipients may be at risk for HBV reactivation due to the lifelong immunosuppression (e.g., corticosteroids, lymphoid-depleting agents, calcineurin inhibitors) required to prevent allograft rejection.
In addition to recipient-related HBV reactivation, there is an added consideration of donor-derived HBV infections. As of 1 March 2021 per U.S. organ procurement organization policy, all potential donors are tested with HBsAg, anti-HBc, and HBV DNA to identify those with CHB or latent HBV. For HBV-negative recipients, the use of these allografts from HBsAg-positive and/or anti-HBc-positive donors confers risk of de novo HBV infection to the recipient, requiring tailored recipient–donor matching, prophylactic strategies, and post-transplant monitoring. This section will focus on screening, risk stratification, and management strategies for HBV in solid organ transplant (SOT) recipients. For a discussion of HBV reactivation in hematopoietic stem cell transplant recipients, we direct the readers to these other reviews [
38,
39,
40].
Post-transplant HBV serologic monitoring for SOT recipients deserves special attention. As the natural course of HBV infection is influenced by both viral replication and host immunity, HBV control in the post-transplant setting is heavily influenced by the degree of immunosuppression. As with the risk of post-transplant infection with Epstein–Barr virus and other opportunistic pathogens, the risk of HBV recurrence or reactivation escalates with higher degrees of immunosuppression [
41]. Therefore, patients should be monitored more closely during periods of more intense immunosuppression (i.e., the first year post-transplant) with laboratory studies that include HBsAg and HBV viral load. If NA therapy fails to fully suppress the HBV DNA level, further evaluation with HBV DNA polymerase testing should be performed to guide tailored adjustments to the treatment regimen. Additional strategies to achieve complete viral suppression should be considered if not achieved with a single agent. These strategies may include combination therapy with two NAs, such as tenofovir/emtricitabine or tenofovir/entecavir. The tenofovir/emtricitabine combination offers a lower-cost, single-pill option, while tenofovir/entecavir may provide greater efficacy by combining two first-line agents.
7.1. Solid Organ Transplantation in Recipients with CHB
Prophylactic treatment should be used to prevent HBV infection of the liver allograft and post-OLT recurrent infection in the HBsAg-positive recipient/HBV-negative donor (
Table 2).
For patients with end-stage liver disease and/or hepatocellular carcinoma due to CHB, an orthotopic liver transplant can be a life-saving intervention. During the transplant surgery, the recipient’s HBV-infected liver is replaced. Yet, there is a risk of HBV recurrence in the transplanted liver allograft due to extrahepatic viral reservoirs in the recipient’s circulation and lymphatic system. Without any prophylaxis, the HBV recurrence rate of HBV has exceeded 80%, resulting in a poor graft and recipient survival rate of only ~50% at 5 years post-OLT [
42,
43].
However, these outcomes are now of historical significance due to advancements in prophylactic strategies. For OLT recipients with CHB, a combination of NA and HBIG therapy has been proven effective in reducing HBV recurrence to <5% while ensuring excellent graft and recipient survival rates [
44]. Nonetheless, maintaining long-term viral suppression is crucial, underscoring the importance of selecting NAs with a high barrier to resistance. Currently, these NAs include ETV, TDF, and TAF. When selecting between these options, it is important to consider common nonhepatic comorbidities in the post-transplant population, such as chronic renal insufficiency and poor bone health. Approximately 20% of transplant recipients develop chronic kidney disease within five years post-OLT, primarily due to calcineurin inhibitors and other nephrotoxic agents [
45]. Preliminary evidence suggests that TDF use is associated with higher rates of nephrotoxicity compared to other NAs [
46,
47,
48]; therefore, those with renal dysfunction may benefit from using ETV or TAF long term. Older agents such as lamivudine, adefovir, and emtricitabine are not recommended due to their high rates of drug resistance, which compromise long-term viral suppression. Higher HV viral levels at the time of transplant have been associated with an increased risk of HBV recurrence [
49]. Thus, all OLT candidates with CHB should receive suppressive NA therapy, with the goal of achieving as low an HBV viral level as possible and reducing the risk of HBV recurrence post-OLT.
In addition to lifelong suppressive NA therapy, many transplant programs incorporate HBIG therapy to prevent HBV recurrence after OLT [
50,
51,
52]. HBIG blocks HBV nucleocapsid entry into hepatocytes and neutralizes circulating HBV via a recipient antibody-mediated immune response. HBIG protocols differ in dose and duration according to the transplant center. Recipients at low risk for recurrence receive HBIG peri-operatively or within the first week post-OLT. However, recipients with risk factors for HBV recurrence (e.g., elevated pre-transplant HBV viral load, history of non-adherence, and baseline NA antiviral resistance) are typically recommended to receive HBIG infusions up until the first year post-OLT. Those who are co-infected with HDV are also recommended an extended course of HBIG, as HBV recurrence invariably leads to HDV co-recurrence, for which there are limited therapies. However, not all programs utilize HBIG due to the added costs and unclear benefits beyond NA monotherapy alone [
44,
52,
53].
Prophylactic treatment is used to prevent HBV reactivation in HBsAg-positive recipients of nonhepatic solid organs.
For nonhepatic (e.g., kidney, pancreas, heart, lung, etc.) solid transplant recipients with CHB, management focuses on preventing HBV reactivation, as the native HBV-infected liver remains intact (
Table 3). Much of the data from nonhepatic SOT recipients are derived from kidney transplant recipients. In renal transplant recipients with CHB, the risk of HBV reactivation without antiviral prophylaxis is markedly elevated, ranging from 50% to 94%, which leads to much poorer recipient survival rates. Thus, to prevent recurrent HBV and its attendant complications, current guidelines recommend lifelong prophylactic antiviral therapy with a high-barrier NA to prevent reactivation and its associated complications. While LAM has historically improved survival in renal transplant recipients, its high rates of resistance limit its long-term use. Thus, high-barrier NAs are preferred due to their long-term efficacy. As with OLT recipients transplanted for CHB, ETV and TAF may be preferred over TDF due to a lower risk of renal toxicity [
47,
48].
Prior to transplant, all SOT candidates with CHB should be evaluated by a provider with expertise in the management of HBV, and NA therapy should be initiated as per guidelines. If NA therapy is not initiated pre-transplant, it should be started at the time of transplant and continued indefinitely, as the risk of HBV reactivation persists as long as immunosuppressive therapy is required. An exception is if immunosuppression is discontinued following graft failure, such as in the case of kidney allograft failure necessitating a return to dialysis.
7.2. Solid Organ Transplantation in Recipients with Latent (Anti-HBc-Positive) HBV
Recipients with latent HBV and who receive an HBV-negative liver allograft typically do not need antiviral prophylaxis due to a minimal risk of HBV recurrence [
54,
55]. The native liver, the main source of HBV recurrence, is removed. These patients undergo HBV viral studies (HBsAg, HBV viral load) every 1–3 months for the first year and then annually afterward.
In contrast to OLT recipients, nonhepatic SOT recipients retain their native liver and thus have a low, albeit substantial (<5%) risk of HBV reactivation. A systematic review and meta-analysis of 16 retrospective cohort studies and 2913 nonhepatic SOT recipients reported an overall HBV reactivation rate of 2.5% [
56]. On subgroup analyses, the reactivation rate was significantly higher in patients who were non-immune (anti-HBs negative; 7.8%) and received lymphoid-depleting therapies (7.3% for recipients who received rituximab, 4.9% for those who received anti-thymocyte globulin (ATG)). Among those with HBV reactivation, complications were frequent and serious; 11% of recipients with HBV reactivation experienced HBV-related graft failure and/or died.
Data regarding the optimal management strategy in this population remain limited. While guidelines generally do not recommend routine prophylactic antiviral therapy [
50,
54,
55], some centers opt for initiating NA prophylaxis in patients with higher-risk profiles, such as those who are anti-HBs negative (non-immune) and/or receiving B-cell depleting therapies, such as rituximab or other lymphodepleting agents, such as alemtuzumab or ATG. In one retrospective cohort study of 180 nonhepatic SOT recipients transplanted at the Mayo Clinic, 77 recipients received prophylactic NA therapy, and 103 recipients did not receive NA prophylaxis [
57]. No recipient who received prophylactic NA therapy experienced HBV reactivation. In contrast, 12 of 97 (12%) of those who did not receive prophylaxis experienced HBV reactivation. HBV reactivation occurred in 29% (2/7) of recipients exposed to rituximab and 100% (2/2) who were exposed to lymphoid-depleting agents, such as ATG or alemtuzumab. Thus, given the excellent side effect profile of first-line antiviral agents, such as ETV and TAF, and the availability of low-cost generic ETV, it may be prudent to consider prophylactic antiviral treatment in this population.
7.3. Solid Organ Transplantation in HBV-Negative Recipients with Anti-HBc-Positive Allografts: Risk of Graft-Related de Novo HBV Infection
The availability of anti-HBc-positive allografts has significantly expanded the donor pool, particularly in HBV-endemic regions, such as Asia and Africa, without compromising recipient or graft survival. While these allografts may be best allocated to recipients with CHB (i.e., recipient HBsAg positive), with the appropriate prophylactic strategies, even HBV-negative (i.e., HBsAg negative) recipients of an anti-HBc-positive liver can achieve excellent outcomes. A retrospective study from Hong Kong described a similar 10-year graft survival rate for 416 recipients of anti-HBc-positive grafts (76.8%) when compared to 548 recipients of anti-HBc-negative grafts (74.8%) and without any difference in graft dysfunction, recipient death, or hepatocellular carcinoma [
58].
However, the utilization of these liver allografts does carry a risk of de novo HBV infection to the recipient via transmission from the transplant allograft. The risk depends also on the recipient’s HBV serologies. Without any prophylaxis, recipients who are HBV naïve/non-immune (anti-HBc negative, anti-HBs negative) are at the highest risk for de novo HBV infection (~48%), followed by recipients who are HBV exposed/non-immune (anti-HBc positive, HBsAg negative, anti-HBs negative; risk~13%) and then recipients who are vaccinated (anti-HBc negative, anti-HBs positive; HBV risk~9%) [
59]. Recipients who are naturally immune (anti-HBc positive, anti-HBs positive; HBV risk ~1%) have the lowest risk of de novo HBV infection.
Many transplant programs administer prophylactic NA to recipients at higher risk of de novo HBV infection (e.g., all recipients except those naturally immune). In a meta-analysis of 26 studies and 462 recipients of anti-HBc-positive liver allografts, NA prophylaxis substantially reduced the risk of de novo HBV infection from 58% to 11% in HBV-naïve/non-immune (anti-HBc negative, anti-HBs negative), 18% to 2% in vaccinated (anti-HBc negative, anti-HBs positive), and 14% to 3% in HBV-exposed/non-immune recipients (anti-HBc positive, anti-HBs negative) [
60]. In this study, NA prophylaxis did not reduce the risk of de novo hepatitis in recipients who were naturally immune (anti-HBc positive, anti-HBs positive). HBIG is not typically utilized as it does not provide additional benefits for preventing de novo HBV infection beyond NA monotherapy in this setting [
59].
The risk of transmission for anti-HBc-positive allografts is mainly observed in OLT recipients; nonhepatic SOT recipients have much lower risks of de novo HBV infection (<1%). In a recently published meta-analysis of 13 studies and 2516 recipients of anti-HBc-positive kidney allografts, only nine (0.36%) cases were reported [
61]. Notably, the risk of HBV infection was significantly higher among recipients who did not receive prophylaxis and lacked immunity, with a rate of 5.71% (2/35) observed in those who were anti-HBc positive and anti-HBs negative. There were no differences in recipient or graft survival for recipients of anti-HBc-positive vs. anti-HBc-negative kidney allografts. Similarly low rates of HBV infection and excellent outcomes have been reported for the use of anti-HBc-positive thoracic allografts, although data are scarce [
62,
63,
64].
All potential SOT recipients who are HBV non-immune should be vaccinated. The optimal prophylactic strategies for recipients of nonhepatic anti-HBc-positive allografts post-transplant remain uncertain, but based on limited data regarding a higher risk of infection for non-immune patients [
61], NA prophylaxis can be considered for those who are HBV non-immune. In the absence of NA prophylaxis, routine post-transplant monitoring, including HBsAg, HBV DNA every one to three months, and on-demand therapy, is recommended.
7.4. Solid Organ Transplantation in HBV-Negative Recipients with HBsAg-Positive Allografts: Acquired Chronic HBV Infection
Liver allografts from donors who are HBsAg positive or HBV nucleic acid testing (NAT) positive are not routinely utilized, as their use typically results in chronic HBV infection in recipients, which invariably results in chronic HBV infection. However, a growing body of literature suggests that HBsAg-positive allografts can effectively expand the donor pool and achieve similar patient and graft outcomes to those of HBsAg-negative ones [
65,
66,
67,
68,
69]. Ali et al. compared clinical outcomes in 209 OLT recipients of HBV-positive allografts (defined as HBsAg positive or HBV NAT positive) to 1045 matched recipients of HBV-negative allografts using data from the Organ Procurement and Transplantation Network (OPTN) database. This study found no statistically significant differences in recipient mortality (3-year survival: 84.8% for the HBV-positive group vs. 82.3% for the HBV-negative group,
p = 0.47) or graft loss (3-year graft survival: 77.9% for the HBV-positive group vs. 79.7% for the HBV-negative group,
p = 0.72). Similar findings were reported using data from the China Liver Transplant Registry [
66]. Among 259 recipients of HBsAg-positive allografts and 259 matched recipients of HBsAg-negative allografts, the investigators observed comparable 3-year survival rates (60.4% vs. 69.1%, respectively,
p = 0.062). These studies highlight the potential for HBV-positive allografts to safely increase the available donor pool without compromising outcomes. Prior to transplant, a thorough histologic evaluation of the donor’s liver was performed to confirm the absence of significant fibrosis. Additionally, grafts from donors with known HDV co-infection should be discarded given the lack of effective therapies for chronic HDV infection [
70].
Transplantation with these HBV-positive liver allografts almost invariably results in chronic HBV infection in the recipient, making long-term suppression with NA therapy essential. High barrier-to-resistance NAs, such as ETV, TDF, and TAF, should be prioritized. If the recipient does not respond adequately to first-line NA therapy, additional strategies to achieve complete viral suppression should be explored, including HBV DNA polymerase sequencing, to identify resistance mutations and tailor therapy accordingly. The role of HBIG is likely limited in recipients of HBsAg-positive liver allografts, as HBIG only prevents HBV infection of the allograft but does not provide any therapeutic benefits once HBV infection has already occurred, as is the case with HBV-positive donor allograft transplant.
Another important consideration in the recipient of an HBsAg-positive graft with resultant chronic hepatitis B infection is the risk of de novo HCC in these immunosuppressed individuals. Given that HBV is a pro-oncogenic pathogen, there is concern for an increased risk of HCC in recipients of HBV-positive allografts. While the absolute risk of HCC in this transplant population is not well defined, adequate viral suppression with NAs has consistently been shown to significantly reduce the risk of HCC in the immunocompetent population [
71,
72]. Given the increased risk of HCC due to the use of immunosuppressants in a post-OLT population, it is important to consider HCC screening for all recipients of HBV-positive allografts with liver imaging (e.g., ultrasound, computed tomography, or magnetic resonance imaging) and alpha-fetoprotein (AFP) every six months. This is particularly critical for recipients of grafts from older donors of Asian or African descent, who may carry an elevated baseline risk for HCC.
The use of nonhepatic organs from HBsAg-positive donors has historically been considered marginal due to the risk of HBV transmission. However, nonhepatic allografts can also be utilized to improve the donor pool and transplant access [
73,
74]. While the liver is the main source of HBV transmission, HBV remains present in the circulation and lymphocytes, presenting a lower but still notable risk of de novo HBV infection [
75].
Preliminary data have demonstrated satisfactory outcomes when recipients are treated with antiviral therapy, with or without HBIG prophylaxis. Delman et al. found that among fifty-six kidney transplant recipients of HBV NAT-positive donors, nine (16.7%) developed de novo HBV infection and active viremia [
67]. All nine patients achieved HBsAg clearance after NA therapy with ETV and none developed any HBV-related complications. Graft and recipient survival rates were excellent, suggesting that effective antiviral therapy can mitigate the risks associated with HBV-positive allografts. Tuncer et al. observed no cases of de novo HBV infection among 35 HBV-immune recipients of kidneys from HBsAg-positive and HBV DNA-negative living donors [
76]. Of interest, neither HBIG nor NA was used prophylactically for the patients in this study. These findings suggest that natural HBV immunity may mitigate the risk of DNH in such cases. Finally, Yin et al. reported on 105 recipients of kidneys from HBsAg-positive, HBeAg-positive, and HBV DNA-positive donors [
74]. Outcomes were compared with those of recipients of kidneys from HBsAg-negative/anti-HBc-positive donors, and graft and survival rates were found to be similar. Four (3.8%) recipients developed an HBsAg-positive de novo infection; all four patients had subsequent conversion to HBsAg-negative status following NA therapy. There is limited data on thoracic organ transplantation involving HBsAg-positive donors, but satisfactory outcomes have also been reported. In a meta-analysis by Yost et al. of heart transplant recipients, 1 of 11 recipients of HBsAg-positive donors developed de novo HBV infection post-transplant, which was managed with lamivudine.
The optimal management of recipients of HBsAg-positive donors is unknown, but the risk of de novo HBV infection likely exceeds that of nonhepatic, SOT recipients of anti-HBc-positive allografts, and the prophylactic strategy should be tailored accordingly [
54,
55]. Prophylactic NA therapy (combined with HBIG therapy for non-immune recipients) should be considered for all nonhepatic SOT recipients. Routine laboratory monitoring should include assessments of HBsAg and HBV DNA, and liver function tests should be considered at least every three months to detect any signs of HBV transmission.