This study evaluated the utility of sGPVI as a local biomarker in hyper-acute ischemic stroke. We examined two different vascular compartments: (i) the ipsilateral ICA, recognized as a critical site of emerging thromboembolism in the context of symptomatic ICA stenosis [3] and (ii) the intracerebral pial vasculature. The pial circulation sustains residual collateral blood flow crucial for maintaining perfusion during LVO prior to EVT but also contributes to a detrimental platelet-driven inflammatory response under conditions of ischemia/hypoxia, which involves GPVI signaling [9,14].
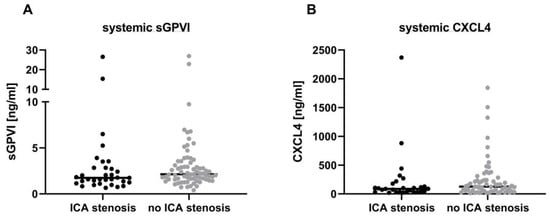
Thromboembolism in patients with symptomatic ICA stenosis is mainly caused by plaque rupture and ensuing exposition of collagen to the circulation [2], which leads to local platelet adhesion involving GPVI as shown in human atheromatous plaques [6,15]. Accordingly, Revacept, a competitive antagonist of GPVI, was applied to patients with high-grade symptomatic ICA stenosis in a recent phase 2 trial to prevent recurrent stroke, but only a combined endpoint of neurological and cardiological events and death reached statistical significance [16]. Despite evidence of local GPVI signaling in the destabilization and rupture of atherosclerotic plaques [3,6], we did not observe differences in systemic arterial sGPVI levels between patients with an ipsilateral ≥ 50% symptomatic ICA stenosis or dissection and stroke patients without a significant ICA stenosis in our present sample. At first glance, this contrasts with findings from Al-Tamini et al., who reported overall increased sGPVI levels in systemic venous plasma samples of acute stroke patients when compared with matched control subjects [17]. Importantly, our analytic approach differed significantly as it utilized arterial blood samples directly from the sites of vascular injury, e.g., the carotid artery, and from pial collaterals provisionally nourishing the ischemic brain under LVO before EVT. This method allowed us to specifically address compartmental GPVI shedding reflecting local platelet responses within the arterial system rather than cross alterations within the venous system. Under LVO before recanalization, pial collaterals maintain some residual retrograde blood flow within the ischemic territory. During EVT, the vessel occluding thrombus is routinely penetrated by a microcatheter, which allows retrieval of minute amounts of blood from the secluded ischemic brain territory before thrombus retraction [13,18]. By analyzing these arterial blood samples, we have shown that there is an accumulation of leukocytes, mainly neutrophils, within this vascular compartment compared with the systemic circulation at the level of the ICA. This local intravascular inflammatory response is partly driven by the chemokines CXCL4 and CXCL7 and the danger-associated molecular pattern HMGB1, which are all released by alpha granules of activated platelets [9,19,20]. Importantly, platelet granule release depends on platelet activation, such as in response to GPVI signaling, but does not mandatorily involve GPVI shedding. We have shown in experimental stroke that the absence of GPVI can mitigate infarct progression in mice before and after recanalization [11]. Based on these findings, we hypothesized that sGPVI may be used as a local intravascular bio-marker for platelet activation in the ischemic brain, but we did not detect overall differences between the paired plasma samples taken at the ICA level compared with the intracerebral pial compartment samples. This suggests that GPVI is not a promising bio-marker, either because it is not locally cleaved and shed into the bloodstream, or due to its rapid clearance once shed. In contrast, CXCL4 levels are also elevated in patients with ICA stenosis, extending our previous findings and further supporting the concept of local platelet activation within the intracerebral vasculature [9].
A particular strength of our study is the presentation of comparative direct human cerebral data obtained during stroke emergency care. There is a high interest in the development of biomarkers for disclosing the underlying stroke etiology and, even more importantly, for the early prediction of outcomes to guide treatment decisions and design add-on-therapies to thrombolysis and/or EVT [21]. Accordingly, using the same approach, we recently showed that levels of neutrophil-derived matrix metalloproteinase-9 within the pial blood vessels predicted bleeding complications and outcomes in AIS even before EVT was performed [22]. In a further search for mechanism-based biomarkers, our present data provide evidence that sGPVI appears not to be suitable as a local stroke biomarker despite the fact that GPVI signaling is critically involved in the pathophysiology of AIS and has emerged as a novel therapeutic target currently under clinical testing [23]. Limitations of our study relate to the limited sample size and its observational design and explorative character, which requires confirmation in larger cohorts. Moreover, since blood is taken during an emergency procedure, no baseline samples for comparison are available.
Source link
Andreas Starke www.mdpi.com