1. Introduction
Antibiotics, as one of the typical emerging pollutants, have gradually aroused a global concern in the last decade for their potential adverse impact on microorganisms, eco-environment, and human health [
1,
2]. Multiple sources are responsible for the extensive presence of antibiotic residues; however, veterinary sources account for more than 52% of the total antibiotic contamination when comparing with human medical treatment and municipal sewage disposal [
3,
4,
5]. For instance, veterinary antibiotics were confirmed the main source of antibiotic residues in the lower-middle reaches of the Yangtze River [
6] and in coastal water of Beibu Gulf [
7]. Admittedly, antibiotics are widely used to prevent disease and to promote the growth of animals. According to a global estimation, the stable growth trend of veterinary antibiotics consumption will continue until 2030, reaching to a total volume of 105,596 ± 3605 tons [
8]. As an important destination for the consumption of veterinary antibiotics, livestock and poultry breeding (LPB) is undoubtedly responsible for the large amount of antibiotics discharge, which needs deeply investigated.
LPB in China has been undergoing a scaling-up transformation from a traditional household production to an intensive industrial production [
9], with an estimated 100,800 tons of veterinary antibiotics consumption every year [
1]. Unfortunately, most antibiotics are difficult to be directly absorbed by the gastrointestinal tract of animals, and approximately 40–90% of them are discharged in their original form or as metabolites in livestock urine and feces [
10,
11]. By the routine application of livestock manure as fertilizers, antibiotics are ultimately released into environmental media through runoff, leachate, or permeation [
12,
13,
14], leading to toxicity, persistence, and bioaccumulation, as well as the development and spread of antibiotic resistance genes (ARG) [
15,
16,
17,
18] and arousing considerable challenge on the environment protection and preservation at the same time. Therefore, it is essential to comprehensively understand the occurrence and evaluate the risk of antibiotics in different livestock excrement, to guide manure application and management for optimal antibiotics prevention.
Generally, quite a lot of veterinary antibiotics are adopted in LPB globally. The three most persistent classes of antibiotics detected in livestock excrement were sulfonamides (SAs), fluoroquinolones (QNs), and tetracyclines (TCs) [
19]. Their concentrations vary significantly by antibiotic class, livestock species, geographical origin, and the type of livestock farms, in the range of a few micrograms to hundreds of milligrams per kilogram [
20]. For instance, SAs in poultry manure obtained a higher concentration as 89 mg·kg
−1 in the U.S., as compared to China with 15.93 mg·kg
−1, while TCs in swine manure were opposite as 37 mg·kg
−1 in the U.S. and 354 mg·kg
−1 in China [
21]. In Europe, SAs (e.g., sulfadiazine) dominated in poultry manure with a higher concentration as 10.1 mg·kg
−1 in Albania and Kosovo, and TCs were more common in cattle manure [
22]. As for swine manure, nine antibiotics were detected with a maximum concentration of 764.4 mg·kg
−1 in Shandong Province, China [
19], whereas Zhu et al. identified 14 different antibiotics in 36 Chinese swine farms at concentrations of 0 to 15.2 mg·kg
−1 [
23]. Although some studies have reported that only low levels of antibiotics remained in manure-based fertilizers, their original contamination in livestock feces or manure have still remained unclear in some regions of China, as well as their related adverse environmental impact.
To date, quite a few Chinese researchers have focused and investigated the original source of antibiotics from livestock excrement in some provinces, including Ningxia [
24], Jiangxi [
25], Liaoning [
26], Shanghai [
27], Zhejiang [
28], Jiangsu [
19], Tianjin [
29] and Shandong [
30], etc. According to above-mentioned literature, TCs, SAs, and QNs were usually obtained a relatively higher level in livestock excrement, which increasing the potential ecological risk to soil or surface water in various degrees. However, as an important LPB industry province in China, no similar reports have been identified for Sichuan yet, where its economy is underdeveloped. Considering the differences in natural geographical regions and the uneven distribution of livestock breeding scale, the occurrence and its associated influence of antibiotics probably exit significant distinction. Hence, it will be of great interest to provide more information about the sources of antibiotics in livestock manure and the surrounding environment media.
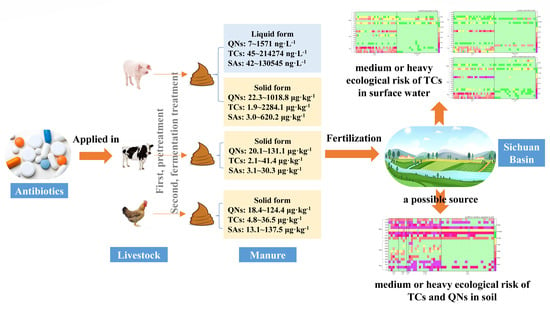
In summary, this study aims to give an exhaustive report on the spatial distribution, source apportionment and ecological risk assessment of 20 antibiotics coming from large-scale livestock farms in Sichuan Basin. The specific goals are as follows: (a) to reveal antibiotics occurrence in soil, surface water, and livestock excrement from different livestock farms and species; (b) to display spatial distribution of antibiotics contamination from different livestock farms by resourceful utilization of livestock manure; (c) to identify potential relationship of antibiotics occurrence between livestock manure and environmental media; (d) to evaluate ecological risks of antibiotics posed by the encouraged utilization of livestock manure as fertilizer.
2. Materials and Methods
2.1. Study Area and Sample Collection
Sichuan Basin, a total area of approximately 260,000 km
2, is mainly located in the central and eastern part of Sichuan Province, covering 17 out of 21 prefecture-level municipal units (
Figure 1), along with relatively developed economy and concentrated population distribution. LPB industry is highly developed in this region, meeting the demand for meat both inside and outside. According to statistical data from Sichuan Provincial Bureau of Statistics in 2022, LPB scales in these 17 municipal units are 37,410,260 swine, 3,169,610 cattle, 8,702,241 sheep and 433,951,911 layers, respectively, accounting for approximately 70% of the total LPB scale (all converted to swine scale) in whole Sichuan (
Table S1). Therefore, the study area is characterized by representativeness and typicality.
In the whole study area, a total of 79 livestock farms were selected and distributed across 17 municipal units in Sichuan Basin, including 64 swine farms, 6 layer farms, 6 cattle farms, and 3 sheep farms, respectively (
Figure 1). From October in 2022 to April in 2023, 20 soil samples, 20 surface water samples, and 86 excrement samples (53 solid forms and 33 liquid forms) were collected by different batches. The locations of sample collection were as follows: all feces samples were collected from manure pits, whereas solid manure samples originated from anaerobic fermentation facilities (swine) and heap piles (cattle, layer, and sheep). Swine liquid manure was obtained from oxidation ponds or ecological ponds used for swine wastewater storage. In addition, all samples were transported to the laboratory within 24 h after collection using refrigerating equipment under 4 °C. Surface water and liquid form samples were analyzed within 48 h, while soil and solid form samples were refrigerated at −20 °C before analysis.
2.2. Target Antibiotics
Totally 20 antibiotics, including 4 TCs, 8 SAs, and 8 QNs were selected and analyzed in this study, which covered the commonly used on livestock farms and frequently detected in livestock excrement and environment [
19,
31,
32]. The full names, acronyms and physicochemical properties of antibiotics are shown in
Table S2. All target antibiotics were purchased from Sigma-Aldrich (Shanghai, China). The purity of all standards was above 98%.
2.3. Sample Pretreatment
The pretreatment for liquid form samples was as follows: 500 mL samples were filtered by 0.7 μm glass fiber membrane. Hydrochloric acid was added to the filtered samples to reach pH around 3.0. Solid phase extraction (SPE) was used by CNW Poly-Sery HLB Pro column. The column was activated sequentially with 5 mL methanol, 5 mL deionized water and 5 mL hydrochloric acid solution with pH = 2.0, then the samples were passed through at a flow rate of 10 mL·min−1. Next, the extraction column was rinsed by 5 mL deionized water, and blown to nearly dry by nitrogen, followed by eluting with 5 mL methanol and 5 mL methanol–acetonitrile mixture (v:v = 1:1). The eluent was finally fixed to a volume of 1 mL with methanol after blowing with nitrogen to nearly dryness, filtered by using 0.22 μm nylon syringe filters, and stored for further analysis.
The pretreatment for the solid samples was as follows: the samples were frozen to dry firstly and then grinded and passed through a 0.25 μm pore size sieve. Next, 1.0 g samples were weighed and added to 50 mL centrifuge tubes. Each tube received 10 mL acetonitrile–McIlvaine buffer and 0.5 g EDTA, followed by vortex mixing. Then, all tubes were ultrasonicated at 80 kHz for 15 min with temperature of 35 °C and centrifuged at 10,000 rpm for 10 min. In addition, 5 mL methanol was added to the residue, followed by vortex mixing, ultrasonication, and centrifugation sequentially. The extraction was repeated a second time following the same steps as above. Both the methanol phase and the acetonitrile phase supernatants were mixed in a new tube, then diluted to 500 mL with ultrapure water. After adjusting pH to around 3.0, the samples were purified and enriched by SPE using CNW Poly-Sery HLB Pro column according to the aqueous pretreatment process.
2.4. Instrumental Analysis
Liquid chromatography–triple quadrupole mass spectrometry (AB SCIEX 3200 Q TRAP LC-MS/MS, USA) was used to analysis antibiotics in samples. The ultra-high performance liquid chromatography BEH C18 1.7 μm column (100 mm × 2.1 mm) was used for separation. The column oven temperature was maintained at 40 °C. The mobile phase A was ultrapure water containing 0.1% (v/v) formic acid and 2.0 mmol·L−1 ammonium acetate, and phase B was chromatographically pure methanol. The flow rate was 0.2 mL·min−1 with a gradient elution program as follows: 0–5 min, 90–85% B; 5–7 min, 85–80% B; 7–10 min, 80–60% B; 10–12 min, 60–40% B; 12–14 min, 40–25% B; 14–16 min, 25–10% B; 16–18 min, 10% B; 18–20 min, 10–90% B; 20–21 min, 90% B. The injection volume was 10 μL.
For all antibiotics, the MS instrument was operated in the electrospray ionization (ESI) positive mode and the data were acquired in the multiple reaction monitoring (MRM) mode. The capillary voltage was 3.0 kV, the source temperature was 150 °C, and the desolvation gas temperature was 500 °C. The cation scanning mode was used to optimize the parameters related to MS/MS analysis. Optimization results are all detailed in
Table S3.
2.5. Quality Assurance and Control (QA/QC)
Stringent quality control procedures were performed throughout the pretreatment and analysis. Quantification of target antibiotics was accomplished by internal indicators, and the relative standard deviation (RSD) was normally below 15%. The standard curve concentration ranges were from 0.01 to 500 μg·L
−1, with linear correlation coefficients above 0.99 (
Table S4). The detection limits of liquid sample were 0.0001, 0.0001, and 0.002 ng·L
−1 for TCs, SAs, and QNs, respectively, whereas the detection limits of solid sample were 0.3, 0.2, and 0.3 μg·kg
−1, respectively.
2.6. Source Apportionment Methods
Principal components analysis (PCA) was adopted to analyze the characteristics of antibiotics in soil, surface water and manure samples. To select the corresponding principal factors, their eigenvalues should be above 1 after normalization method [
33]. Data analysis and processing were performed using Origin 2017 software.
2.7. Potential Ecological Risk Assessment of Antibiotics
The risk quotient (RQ) approach was usually used to evaluate the potential ecological risk of antibiotics to ecosystems [
19,
29,
33]. The RQ values were divided into four levels of risk: (a) insignificant risk (<0.01), (b) low risk (0.01–0.1), (c) medium risk (0.1–1), and (d) high risk (>1). The RQ calculation formula is as follows:
where MEC represents the measured environmental concentration of antibiotic and PNECwater/soil represents the predicted no-effect concentration of antibiotic on aquatic or terrestrial creature.
Based on classical ecotoxicological tests, species sensitivity distributions (SSD) was recommended to derive specific
PNECwater of antibiotics in this study [
34], which estimating the hazardous concentration (HC
5) corresponding to a certain proportion (usually under 5%) of numerous EC
50 or LC
50 values of individual species when they are adversely affected [
35]. In addition, according to the Technical Guidance Document (TGD) on Risk Assessment [
36], the
PNECsoil of antibiotics could be calculated through formulas from (2) to (6).
where AF represents the assessment factor (this study takes the value as 1); Ksoil-water represents the soil–water partition coefficient, m3·m−3; RHOsolid represents solid density (the value defined in TGD is 2500 kg·m−3); Fwater-soil and Fsolid-soil represent the volume fractions of the water and solid phases in the soil, respectively (the values defined in TGD are 0.2 and 0.6 m3·m−3, respectively); Kpsoil represents the solid phase–water partition coefficient, L·kg−1; Focsoil represents the mass fraction of organic carbon in soil solids (the value defined in TGD is 0.02 kg·kg−1); Koc represents the organic carbon–water partitioning coefficient; Kow represents the octanol–water partition coefficient.
The
PNECwater and
PNECsoil values of antibiotics for different species are provided in
Table 1. All EC
50 or LC
50 data from classical ecotoxicological tests in this study were obtained from Environmental Protection Agency (EPA) ECOTOX database (
https://cfpub.epa.gov/ecotox/, accessed on 4 November 2024).
4. Conclusions
This study provides a comprehensive investigation to illustrate the occurrence of 20 target antibiotics from large-scale livestock farms and environmental media (soil and aquatic water) in Sichuan Basin, China. Three categories of antibiotics were all detected with significantly varied concentrations among different livestock farms and different livestock species. QNs and TCs were ubiquitous in livestock excrement, where TCs obtained the highest average concentrations as 863 and 235 μg·kg−1 in swine farms, QNs obtained the highest average concentrations as 3155 and 76 μg·kg−1 in layer farms, and as 76 and 75 μg·kg−1 in cattle farms. Spatial distribution reveals that central, south, and southwest of Sichuan Basin displayed a higher contamination of antibiotics from livestock manure, which were considered as a possible source for their relatively higher abundance detected in soil according to source analysis, while other sources were responsible for the detection of antibiotics in surface water. The ecological risk assessment indicates that the resourceful utilization of livestock manure as fertilizer would probably elevate risk to a high level caused by antibiotic exposure to sensitive organisms living in soil and aquatic water, especially TCs and QNs from swine manure. However, the use of antibiotics in livestock farming is inevitable, and preventing and solving antibiotic contamination will require better management and further research.