1. Introduction
The
Pulicaria genus (Asteraceae) includes eighty-two accepted species distributed in three different continents, Africa, Asia, and Europe, but the species are mainly concentrated in the Mediterranean basin region [
1].
Interesting biological properties, such as cytotoxic [
2], antibacterial [
3], anti-inflammatory [
4], antihistaminic [
5], antifungal [
6], insecticide [
7], and leishmanicidal [
8], have been assessed for several
Pulicaria species.
Pulicaria species are commonly employed in folk medicine. In fact, the biological potential of some species such as
P. cruncha and
P. incisa used in the culinary field as drinks [
9,
10,
11] or as food flavorings has been reported [
11]. Some of these are also exploited to intensify the flavor or aroma of an infusion such as
P. undulata [
11] or
P. jaubertii E. Gamal–Eldin [
12,
13].
Inula arabica, synonymous with
P. arabica (L.) Cass, in turn, is used to relieve swelling and painful boils [
14]. Other
Pulicaria species, known in Iran as “kak kosh” and “shebang”, are commonly utilized as herbal teas, flavoring agents, and ethnobotanical remedies for inflammatory diseases and to treat severe heatstroke and diarrhea [
15].
P. odora L. is a Moroccan medicinal, locally known as “Ouden El hallouf”; its properties, as traditional medicine, includes the treatment of back pain, cramps, and menstrual pain [
16].
Different secondary products such as phenolic compounds, terpenoids with different skeletons, diterpenes, triterpenoids, have been identified in several
Pulicaria species [
17]. Their EOs, extracted from leaves, fruits, and even barks, have also been tested as potential anticancer, antidiabetic, and anti-inflammatory agents, and their composition was recently reported [
18].
According to Euro+Med PlantBase [
19], twelve
Pulicaria taxa grow in Morocco and
Pulicaria burchardii Hutch. subsp.
burchardii is one of them. This taxon, endemic of Morocco, Mauritania, and Fuerteventura (Canary Islands), is one of the two subspecies of
P. burchardii Hutch. The other,
P. burchardii subsp.
longifolia E. Gamal-Eldin, is endemic of Cape Verde [
1].
P. burchardii Hutch. subsp.
burchardii is a very rare shrub with a hemispherical shape, densely branched, with spreading lateral branches that can root in the substrate. The tomentum is short and dense, and white in branches and leaves. The leaves are alternate, linear-spatulate, 1–3 cm long. The capitula are terminal (up to 1.5 cm in diameter) with yellow ligules and florets. It is a halo-psammophilous species, although it is capable of thriving on stony soils or even with calcareous crusts, and grows in places exposed to the sea wind and forms micro-dunes around it when the ground is sandy, similar to other species in its habitat [
20].
The only previous phytochemical report on this taxon concerns the isolation, from the ethanolic extract of the aerial parts of the accession from Canary Island, of seven known compounds including triterpenoids, 3
β-hydroxytaraxaster-20-en-30-al, 11-oxo-aamyrin, vanillin, 4-hydroxybenzoic acid, scopoletin, stigmasterol, and
β-sitosterol [
21]. On the other hand, nothing has been published on its EO (PBB) or on its biological properties.
EOs represent a promising phytochemical frontier with high antiviral and antibacterial efficacy against several species. The chemical diversity of bioactive molecules in EOs makes them a new potential source of antiviral drugs, which could overcome the limitations associated with current synthetic drug treatments. In detail, it has been documented that EOs from oregano, cloves, and
Melaleuca alternifolia have shown activity against
Herpes simplex virus type-1 (HSV-1), adeno virus type-3, coxsackie virus B-1, and the polio virus [
22,
23]; phenylpropanes, sesquiterpenes, and triterpenes, components of EOs, have been found to act against HSV-1, poliovirus, and the influenza virus [
24,
25,
26]. However, to date, no study has investigated the antiviral, antioxidant, and antibacterial potential of the essential oil derived from
P. burchardii subsp.
burchardii. In this context, the present investigation evaluated the efficacy of PBB against HSV-1 and SARS-CoV-2, representing viral models with DNA and RNA genomes, respectively. The analysis was also conducted at different stages of viral infection to better understand the PBB mechanism of action. Furthermore, the possible anti-germinative actions of PBB on both
B. subtilis and the food pathogen
B. cereus were studied to evaluate its possible use as a natural food preservative. The particular composition of PBB, essentially consisting of an abundant and primary component 1-oxo-bisabolone, a rare compound, makes it a unique and interesting candidate for many applications.
3. Discussion
The presence of 1-oxo-bisabolone in so large an amount is quite interesting. In fact, it has been identified in a small amount (2.5%) in the EO of
Pulicaria gnaphalodes (Vent.) Boiss. [
29]. In the literature, there are quite a few papers reporting the occurrence of 1-oxo-bisabolone from a natural source. The first report of its presence (15%) in
Stevia purpurea Pers was made by [
30]. Later on, it was reported in the EOs of two Poaceae:
Cymbopogon citratus Staff [
27] and
Cymbopogon distans (Steud.) Wats. [
31]. A good quantity of 1-oxo-bisabolone was detected in the leaf EO of
Commiphora africana (A. Rich.) Engl [
28], whereas the EO of the two Asteraceae,
Asteriscus graveolens (Forssk.) Less [
32] and
Geigeria alata (DC.) Oliv.
et Hiern. [
33], contained limited amounts. Finally, its presence in the EO of
Rhododendron thymifolium Maxim (Ericaceae) and its insecticidal properties were recently determined [
34]. Although 1-oxo-bisabolone has been detected in only one
Pulicaria taxa, some bisabolene derivatives have been identified in the EOs of some species such as
β-bisabolol in
P. gnaphalodes (Vent.) Boiss. [
29],
α-bisabolol in
P. somalensis O. Hoffm. [
35],
P. undulata (L.) C. A. Mey [
36,
37], and
β-bisabolene in
P. vulgare ssp.
graeca (Sch.-Bip.) Fiori [
38].
Specifically, 1-oxo-bisabolone is an oxygenated sesquiterpene derived from bisabolone, a compound found in many medicinal plants. For instance, chamomile is one of the most well-known medicinal plants containing sesquiterpenes including various bisabolone oxides [
39]. Chamomile extracts have also demonstrated antimicrobial properties against both Gram-positive and Gram-negative bacteria. Tocai-Moțoc et al. [
40] highlighted that the EO obtained from
Matricaria chamomilla L. flowers, rich in bisabolol oxide, bisabolol, and farnesene, exhibited significant antimicrobial activity against
E. coli and
S. aureus.
Avonto et al. demonstrated that in extracts of German
Matricaria chamomilla, used as a medicinal plant in Western herbal medicine, bisabolol and its oxidized metabolites served as marker compounds for distinguishing different chemotypes. These compounds contribute to the therapeutic properties, such as anti-inflammatory, antibacterial, insecticidal, and anti-ulcer, of this species [
41].
Sage is another plant that contains sesquiterpenes including compounds similar to 1-oxo-bisabolone. Sage extracts have been studied for their antimicrobial properties. Paknejadi et al. demonstrated that the EOs derived from five different species of sage showed significant antimicrobial activity, particularly against Gram-positive strains such as
S. aureus [
42].
Other EOs have also demonstrated notable antimicrobial activities attributable to the presence of sesquiterpenes. Castagliuolo et al. demonstrated that the EO of
Thymus richardii subsp.
nitidus exhibited significant antimicrobial activity, especially against Gram-positive strains, attributable to the presence of thymol and
β-bisabolene [
43]. The results of our study are consistent with the existing literature, highlighting that 1-oxo-bisabolone is likely the primary agent responsible for the antimicrobial activity of PBB. We observed a higher susceptibility to these compounds in Gram-positive strains. Both the plate inhibition assay and the viable count assay indicated that PBB exhibited higher activity against
S. aureus and
B. subtilis compared to
E. coli and
P. aeruginosa. Since
B. subtilis initially proved to be the most sensitive strain, we decided to further investigate the antimicrobial activity against other bacilli, such as
B. cereus and
B. licheniformis, by calculating the MIC. These bacilli showed surprisingly low MIC values of 6 and 5 mg/mL, respectively, with bacteriostatic potential.
The precise mechanism by which EO exerts its antimicrobial properties is not always fully understood. Considering the large number of different chemical compounds present in EOs, it is likely that their antibacterial activity is not due to a single specific mechanism, but rather to multiple cellular targets. Cena stated that EOs, in general, can destabilize bacterial cell membranes, causing the loss of cellular contents and consequently bacterial death. However, their antimicrobial activity is likely due to the presence of various molecules in their composition that work synergistically, acting on multiple target sites within the bacterial cell [
44]. In our case, it was evident that the target was not the cell membranes, as the DAPI/PI double staining showed a marked blue fluorescence from DAPI, associated with a decrease in the number of cells. This indicates that the cell membranes were intact and suggests that the mechanism of action may involve other processes such as the inhibition of protein or DNA synthesis. Thus, PBB, by interfering with crucial biosynthetic processes for microbial survival, could exert its antimicrobial activity. In investigating the antibacterial mechanisms associated with
α-bisabolol, Cruz et al. demonstrated that
α-bisabolol is capable of inhibiting the efflux pumps TetK and NorA, responsible for pumping antibiotics into the extracellular space [
45]. Unsubstituted sesquiterpenes like
α-humulene non-specifically target the membrane of Gram-positive bacteria, increasing permeability and the loss of intracellular contents [
46]. Introducing a lactone fraction into sesquiterpenes enhances their activity against filamentous fungi, as seen with costunolide, cynaropicrin, deacetylxanthumin, and isoalantolactone [
47].
Various EOs, in addition to possessing antimicrobial, antioxidant, and antiviral activities, also exhibit anti-germinative properties. Sakai et al. demonstrated that the germination times of
B. subtilis spores in a medium containing carvacrol and thymol were delayed. Specifically, the addition of carvacrol and thymol inhibited the germination of
B. subtilis spores, with thymol exhibiting a more pronounced degree of inhibition than carvacrol [
48]. PBB also showed anti-germinative capacities for the spores of
B. subtilis and
B. cereus. Specifically,
B. subtilis spores treated with PBB at a concentration of 0.6 mg/mL exhibited an initial block in germination, followed by a significant slowdown compared to the controls. Similarly, the germination of
B. cereus spores treated with a concentration of 5 mg/mL also showed a deceleration in germination.
Plant-derived substances also offer a promising avenue for the discovery of lead compounds that can guide the development of new antiviral agents [
49]. Among the vast array of plant-based compounds, EOs have gained significant attention in recent years for their antimicrobial properties. These EOs, which constitute an important component of the plant’s chemical defense system, are increasingly being evaluated for their potential to combat various pathogens including viruses [
50]. EOs are complex mixtures of secondary metabolites including terpenes, phenylpropanoids, and other aromatic compounds, traditionally used for their therapeutic properties [
51]. Their potential as antiviral agents have been explored through numerous studies, evaluating their effectiveness in inhibiting viral replication, interrupting viral entry, and interfering with viral assembly [
52]. In this context, the present study aimed to evaluate the antiviral potential of PBB against SARS-CoV-2 and HSV-1, two viruses with markedly different envelope compositions and genome organizations. This evaluation provides valuable insights into the potential of PBB as a broad-spectrum antiviral agent. To determine the range of non-toxic concentrations for the antiviral evaluation of PBB, cytotoxicity assays were conducted on VERO-76 cells using concentrations ranging from 5 mg/mL to 0.0078 mg/mL. PBB exhibited 50% cytotoxicity at a concentration of 10 mg/mL. However, at concentrations starting from 0.31 mg/mL, its cytotoxicity was less than 10%. Based on these findings, PBB was chosen for further antiviral assays. Döll-Boscardin et al. reported that the EOs of
Eucalyptus benthamii exhibited CC
50 values between 108.33 μg/mL and 54.96 μg/mL, respectively, on Jurkat cells. For J774A.1 cells, the CC
50 values were 287.98 μg/mL and 252.55 μg/mL, while for Hela cells, EOs induced 50% cytotoxicity at concentrations of 84.24 μg/mL and 110.02 μg/mL, respectively [
53]. Tavakoli et al. assessed the cytotoxic potential of EOs from the aerial parts of
Ferulago trifida on the MCF7, A-549, and HT-29 cell lines. The recorded CC
50 values were 22.0 μg/mL, 25.0 μg/mL, and 42.55 μg/mL, respectively [
54]. These studies demonstrate that cytotoxicity varied not only with plant maturation, but also with the cell line used. To date, the Food and Drug Administration (FDA) has approved over 90 antiviral drugs. However, the increase in drug-resistant viral strains highlights the need for new broad-spectrum antiviral drugs. EOs have shown potential in antiviral research, offering a natural alternative or complement to traditional therapies. In this study, we report the antiviral activity of PBB against SARS-CoV-2 and HSV-1 within a concentration range of 0.31 to 0.039 μg/mL. PBB demonstrated virucidal action against HSV-1 and SARS-CoV-2, with CC
50 values of 45% and 49%, respectively. We hypothesize that PBB alters the viral surface, thereby preventing the initial infection phases of attachment and fusion of the virus to the host cell. Several studies have documented the virucidal action of EOs, highlighting their potential as antiviral agents. Ribas Pilau et al. evaluated the antiviral activity of
Lippia graveolens EO against HSV-1. At a concentration of 99.6 μg/mL, the EO inhibited viral infectivity by 50%. This inhibition was attributed to alterations in the viral envelope structure, which subsequently influenced the primary phase of viral infection [
55]. Elaissi et al. evaluated the antiviral potential of
Eucalyptus EO against Coxsackie virus B3. The EO demonstrated an IC
50 value of 0.7 mg/mL in the previrus assay. This suspension also affected the attachment and fusion phases of the viral particle with the host cell [
56]. Ćavar Zeljković et al. demonstrated the virucidal potential of
Mentha aquatica EO against SARS-CoV-2, where 50% of the viral particles showed structural alteration of the viral envelope at a concentration of 189.73 mg/L [
57]. Similarly, Haddad et al. reported the virucidal action of EO, and their research demonstrated that the
Ayapana triplinervis EO, at a concentration of 38 μg/mL, reduced the infectivity of the Zika virus by 50% and effectively destroyed the viral envelope [
58]. While the reported studies shared the observation of the virucidal action of EOs, the active doses were different. The latter were influenced by the composition of the EO, and above all, by the viral species studied. The compounds contained in PBB, and in particular 1-oxo-bisabolone, are very interesting and can be studied in the future for potential biological applications, hoping to lead to the development of new drugs or pesticides.
4. Materials and Methods
4.1. Plant Materials
The fresh aerial parts (stems and leaves) of Pulicaria burchardii Hutch. subsp. burchardii were collected near Chichaoua (Morocco), (31°32’53” N; 8°48’39” E 378 m.s.l.), in May 2023. A voucher of this plant, identified by Prof. Ilardi, was deposited at the herbarium in the university complex of Palermo (Vouchers No. PAL 109774).
4.2. Isolation of Volatile Components and Spectroscopical Analyses
For the extraction of the EO, the procedure reported in Lauricella et al. [
59] was performed. The aerial parts, freshly ground, were subjected to hydrodistillation using the Clevenger apparatus following the procedural indications of the European Pharmacopoeia [
60]. Once the EO was obtained, it was dried and stored at −20 °C until analysis; the sample yield was 0.1% (
w/w). The NMR spectra were acquired with a Bruker Avance II instrument (400 MHz for
1H-NMR and 100 MHz for
13C-NMR) [
61] using chloroform (CDCl
3) as the deuterated solvent with 7.27 and 77.0 ppm as the peak references for the H- and C-NMR spectra, respectively.
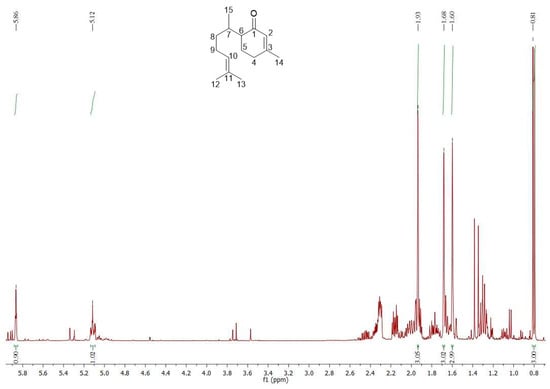
δC (ppm) 15.57 (C-15), 17.62 (C-12), 22.35 (C-5), 24.08 (C-14), 25.68 (C-13), 25.99 (C-9), 30.26 (C-7), 30.89 (C-4), 34.69 (C-8), 49.84 (C-6), 124.90 (C-10), 127.15 (C-2), 131.38 (C-11), 161.09 (C-3), 201.07 (C-1).
4.3. GC and GCMS Analyses
Analysis of PBB was performed according to the procedure reported by Gagliano Candela et al. [
62].
4.4. Bacterial Strains
The antimicrobial effect was assessed using the following bacterial strains. Gram-negative: Escherichia coli DH5α and Pseudomonas aeruginosa PAOI ATCC 15692; Gram-positive: Staphylococcus aureus ATCC6538P, Bacillus subtilis AZ54, Bacillus cereus ATCC10987, and Bacillus licheniformis DSM8782.
4.5. Antimicrobial Assay
The presence of potential antimicrobial compounds in the essential oil of
P. burchardii was investigated using the agar diffusion method, based on the Kirby–Bauer protocol with some modifications [
63].
Three different volumes (5, 10, and 15 µL) of PBB were placed on Luria–Bertani agar plates overlaid with 10 mL of soft agar (0.7%) and pre-mixed with 10 μL (5 × 10
5 CFU/mL) of
E. coli DH5α,
S. aureus ATCC6538P, and
B. subtilis AZ54 grown for 24 h at 37 °C. The negative control was represented by dimethyl sulfoxide 80% (DMSO) used to resuspend the samples; the positive control was the antibiotic ampicillin (10 µL) concentrated at 0.1 mg/mL. After incubation at 37 °C, antimicrobial activity was calculated based on the diameter of the inhibition zone as arbitrary units per milliliter, according to the equation shown by Bhaskar et al. [
64].
Another method to evaluate antimicrobial activity involved counting the cell viability of the Gram-positive and Gram-negative strains. The bacterial cells (5 × 10
5 CFU/mL) were incubated with PBB at a concentration of 0.1, 0.25, 0.5, 1, 2, 4, 8, and 16 mg/mL. The percentage of bacterial survival was calculated following the protocol shown by Castagliuolo et al. [
43]. Each experiment was conducted in triplicate and the results reported were an average of three independent experiments.
4.6. Determination of Minimal Inhibitory Concentration
The minimum inhibitory concentrations (MICs) of PBB against all bacterial strains were determined using the microdilution method as outlined by the Clinical and Laboratory Standards Institute (CLSI). In brief, ~5 × 105 CFU/mL was added to 95 µL Mueller-–Hinton broth (CAM-HB; Difco) with or without PBB at different concentrations (0.1–16 mg/mL). Following overnight incubation at 37 °C, the MIC100 values were identified as the lowest concentration at which no visible bacterial growth was observed. After that, 5 µL of each sample at the MIC value was subsequently added to 95 µL of fresh medium in 96-well plates and re-incubated at 37 °C for 24 h. Subsequently, we evaluated whether the antimicrobial activity of PBB was bacteriostatic or bactericidal by reading the OD at 600 nm. If there was bacterial growth, the action was bacteriostatic, and if there was no growth, the action was bactericidal. All experiments were conducted in triplicate, and the results were presented as the average of three independent trials.
4.7. Fluorescence Microscopy Experiments: DAPI/PI
For the fluorescence microscopy experiments, two dyes were used: DAPI (4’,6-diamidino-2-phenylindole dihydrochloride; Sigma Aldrich, Milan, Italy) and IP (propidium iodide; Sigma Aldrich, Milan, Italy). Briefly, 100 µL of bacterial cultures of
E. coli DH5α,
S. aureus ATCC6538P, and
Bacillus subtilis (grown to mid-log phase) were incubated in the dark for 2 h at 37 °C with shaking, with or without PBB. After incubation, 10 µL of the bacterial culture was mixed with a solution of DAPI [1 µg/mL] and PI [20 µg/mL]. Samples were examined with an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan) equipped with a DAPI filter (excitation/emission: 358/461 nm). Standard acquisition times for DAPI/PI dual staining were set at 1000 ms. Images were acquired using an Olympus DP70 digital camera, following the method described by Pizzo et al. [
65].
4.8. Spore Production and Purification
Sporulation of
B. subtilis was induced using the nutrient depletion method [
66]. In summary, after 30 h of growth at 37 °C with vigorous shaking in Difco sporulation medium (DSM) (composition per 1 L: 8 g Nutrient Broth, 1 g KCl, 1 mM MgSO
4, 1 mM Ca(NO
3)
2, 10 μM MnCl
2, 1 μM FeSO
4, from Sigma-Aldrich, Germany), spores were observed under an optical microscope, washed four times with cold, sterile distilled water, and centrifuged at 8000×
g for 20 min. The spore purification process involved treatment with 1 M KCl, 10 mM lysozyme, 1 M NaCl, and 0.05% SDS, followed by several water washes [
67].
For B. cereus, sporulation was promoted by inoculating the strain into DSM sporulation medium and incubating at 25 °C with vigorous shaking for 72 h. Mature spores were washed four times with cold, sterile distilled water and further purified by incubating in H2O at 4 °C overnight to lyse any remaining sporangial cells. The purity of both B. subtilis and B. cereus spore preparations was confirmed by microscopy, with samples considered pure when the sporangial cells comprised less than 5% of the observed content.
4.9. Spore Germination Assay
The initial A 600 nm of both B. subtilis and B. cereus spore suspensions was 0.3, corresponding to approximately 4 × 107 spores mL−1.
Purified
B. subtilis spores were activated by heat (20 min at 70 °C) and diluted in 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM glucose, 1 mM fructose, and 10 mM KCl (GFK) in the presence or absence of PBB at a concentration of 0.6 mg/mL. Following activation, germination was initiated by introducing 10 mM asparagine into the mixture, and the optical density at 600 nm was recorded every 10 min over a 60 min period [
68].
Germination of
B. cereus spores was induced by heat (70 °C for 20 min) in a germination buffer of 10 mM Tris–HCl at pH 7.4, 10 mM NaCl [
69] in the presence or not of PBB at a concentration of 5 mg/mL, and subsequently supplemented with 10 mM L-alanine. The drop rate of A 600 nm was measured at 10 min intervals for 90 min.
As further controls, the germination of B. subtilis and B. cereus spores was evaluated in the presence of DMSO as the negative control and PBB as the positive control.
4.10. ABTS Assay
This assay, which evaluates the ABTS radical scavenging activity, was carried out following the protocol outlined by Napolitano et al., with some modifications [
70]. Specifically, 1 mL of ABTS solution was mixed with 100 µL of PBB at concentrations of 1, 2, 4, and 8 mg/mL. Absorbance was measured at 734 nm against a blank, and the percentage of ABTS radical inhibition was calculated using the formula presented in their study. Each experiment was performed in triplicate and the result reported was an average of three independent experiments.
4.11. DPPH Radical Scavenging Assay
The DPPH (2,2-diphenylpicrylhydrazyl hydrate) radical scavenging activity was assessed as described in the literature [
71]. Various concentrations of PBB (1, 2, 4, and 8 mg/mL) were prepared in 100% methanol. Freshly prepared DPPH solution (0.1 mM) was added to the PBB to a final volume of 1 mL, ensuring that the initial absorbance of the DPPH solution was ≤1.0. The reaction mixtures were incubated at 25 °C for 30 min in the dark. Absorbance was measured at 517 nm using a spectrophotometer. The scavenging activity was calculated using the equation: DPPH radical scavenging activity (%) = (1
− AS/AC) × 100, where AS is the absorbance of the reacted mixture of DPPH with the extract sample, and AC is the absorbance of the DPPH solution.
4.12. Cell Viability Assay
The MTT-based cytotoxicity assay of PBB in the HaCat cell lines was determined by a rapid colorimetric assay. In this assay, 20,000 cells were seeded in 96-well microplates and incubated for 24 h at 37 °C, 5% CO2 and humidified air. Then, different concentrations of PBB were added to each well. For the positive and negative controls, H2O2 (1.5 µM) and DMSO were used, respectively. The plates were incubated for 24 h in the same condition. To assess the cell survival, 10 µL of MTT in 90 µL of DMEM solution was added to each well, and the plates were incubated at 37 °C for 4 h. Then, the media were removed and 100 µL of DMSO was added to each well and incubated for 10 min. Absorbance was measured at 570 nm using a Synergy H4 Hybrid Microplate reader (Agilent, Santa Clara, CA, USA). Each concentration was assayed in four wells and repeated three times. Cell viability was calculated based on the untreated cells (DMSO) that were assumed as 100%.
4.13. Cytotoxicity Assessment
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to evaluate the PBB cytotoxicity after exposure to the African Green Monkey (
Cercopithecus aethiops, VERO-76) cell line. Cells were seeded and cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco; Thermo Fisher Scientific, Waltham, MA, USA), adding 2 mM L-glutamine, 100 IU/mL penicillin-streptomycin solution, 4.5 g/L glucose, and 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Waltham, MA, USA). The assay was performed in a 96-well plate, and incubated at 37 °C in a humidified environment with 5% CO
2 for 24 h. Then, cells were exposed to increasing concentrations of PBB (0.078–5 mg/mL) for 24 h. The solvent in which the compound was dissolved (1% DMSO) and 100% DMSO served as the negative (CTRL-) and positive (CTRL+) controls, respectively. After exposure, the compound/medium mixture was removed and each well was treated with MTT solution (Sigma-Aldrich, St. Louis, MI, USA) (0.3 mg/mL) for 3 h at 37 °C. A volume of 100 μL of 100% DMSO was added to allow for formazan crystal solubilization and the absorbance at 570 nm was measured by a microplate reader (Tecan, Männedorf, Switzerland) to calculate the percent cytotoxicity as follows:
4.14. Antiviral Activity
The antiviral properties of PBB were investigated against HSV-1 (enveloped virus with DNA genome) and SARS-CoV-2 (enveloped virus with RNA genome). Both viruses were propagated and titrated by exposing the virus to VERO-76 cells at a multiplicity of infection (MOI) of 0.01. The plaque reduction assay was conducted in different conditions: co-treatment, virus pre-treatment, cell pre-treatment, and post-treatment. The assays were performed in a 12-well plate with the cells seeded at 2 × 105 cells/well for 24 h at 37 °C with 5% CO2 and treated as follows:
Co-treatment: PBB in the concentration range of 0.039–0.31 mg/mL was co-exposed to the cell monolayer with the viral suspension at 2 × 103 plaque-forming units (PFU)/mL for 1 h at 37 °C;
Virus pretreatment: The viral suspension at 2 × 104 PFU/mL was incubated with the compound in the same concentration range for 1 h at 37 °C. Then, a 1 to 10 dilution of the mixture was performed, and the cell monolayer was infected for 1 h at 37 °C;
Cell pretreatment: The cell monolayer, prior exposed to the sample for 1 h at 37 °C, was then infected for another hour with the viral suspension at 2 × 103 PFU/mL;
Post-treatment: Cells first infected with the virus for 1 h at 37 °C were then exposed for another hour to PBB.
After the conditions described, cells were covered with a culture medium supplemented with 5% carboxymethylcellulose to limit the infection spread: 24 h for SARS-CoV-2 and 48 h for HSV-1. To evaluate the cytopathic effect, cells were fixed with 4% formaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA). Plaques were counted and correlated with those coinciding with CTRL− to obtain the percentage of viral inhibition according to the following formula:
For the CTRL+, HSV-1 melittin (5 µM) was used in the co-treatment and in the virus pre-treatment, dextran sulfate (1 µM) in the cell pre-treatment, and acyclovir (5 µM) in the post-treatment, while unexposed infected cells represented the CTRL−. For SARS-CoV-2, PBB (10 μg/mL) was used in the co-treatment and pre-treatment of the virus, ivermectin (10 μM) in the cellular pre-treatment, and remdesivir (10 μM) in the post-treatment, while unexposed infected cells represented the CTRL−.
4.15. Statistical Analysis
Cytotoxicity and antiviral tests were performed in biological duplicates and the results were reported by calculating the mean and standard deviation (SD). Ordinary one-way ANOVA, Dunnett’s multiple comparison test, the cytotoxic concentration at 50% (CC
50), and inhibitory concentration at 50% and 90% (IC
50–IC
90) were calculated using GraphPad Prism software ver. 9 for macOS (GraphPad Software, San Diego, CA, USA,
www.graphpad.com, accessed 9 January 2024). Dunnett’s multiple comparison tests expressed the significance of differences between the PBB-exposed samples compared with the unexposed samples (CTRL−). Values were considered significant at a
p-value < 0.05. Cytotoxicity assays on the human cells were conducted in triplicate (
n = 3), and statistical analysis was calculated using two-tailed paired t-test (*
p < 0.01, ***
p < 0.001).
5. Conclusions
This exploratory study revealed the therapeutic potential of Pulicaria burchardii Hutch. subsp. burchardii essential oil. This sample, investigated using chromatographic (GCMS) and spectroscopic (NMR) techniques, was mainly made up (65.09%) of an oxygenated sesquiterpene such as 1-oxo-bisabolone. Concurrently, the antimicrobial, anti-germinative, antioxidant, and antiviral properties were investigated. The antimicrobial activity, which does not act directly on bacterial membranes, was evaluated on several bacteria, both Gram-positive and Gram-negative, such as E. coli, P. aeruginosa, S. aureus, B. subtilis, B. cereus, and B. licheniformis, showing the highest activity against the B. subtilis strain (MIC 0.6 mg/mL). The antibacterial capabilities against the Bacillus genus were also confirmed by the already excellent anti-germinating properties at the 0.6 mg/mL dose with a deceleration in germination confirmed for up to 90 min of treatment against B. subtilis bacteria. For antiviral activity, the essential oil was tested against some pathogenic viruses including SARS-CoV-2 and HSV-1. In the co-treatment assay, at the highest sub-toxic concentration tested (0.31 mg/mL), the essential oil achieved viral inhibition rates of 69% and 65% for HSV-1 and SARS-CoV-2, respectively, and showed antiviral effects in the extracellular environment, suggesting the inhibition of viral contact and fusion with the host cell. In this phase, the total inhibition of HSV-1 was recorded at 0.31 mg/mL and a 78% reduction against SARS-CoV-2, suggesting a possible virucidal effect of the essential oil. The marked antibacterial and antiviral effects suggest, in a future perspective, an in-depth analysis of 1-oxo-bisabolone, to confirm all the observations made thus far.