1. Introduction
Ice plant (
Mesembryanthemum crystallinum) is a plant that grows natively in Southern Africa, and is capable of withstanding dryness and salt. It is a facultative halophyte recognized for its potential efficacy in preventing lifestyle-related diseases. The possible biological activity of ice plant in preventing and treating metabolic disorders such as diabetes has been acknowledged [
1]. Ice plant has drawn much interest as it contains a broad and diverse range of natural compounds with various biological and pharmacological effects [
2].
Numerous investigations have revealed that polyols such as D-pinitol are abundant in ice plant. Several studies have demonstrated that D-pinitol is present at the highest levels among cyclitol molecules, whereas chiro- and myo-inositol contents are comparatively low [
2,
3]. D-pinitol inhibits rat breast carcinogenesis [
4] and exhibits antidepressant effects [
5]. In addition, the anti-inflammatory and antioxidant qualities of D-pinitol have been extensively investigated [
6]. In numerous studies on diabetes, in rats with diabetes caused by streptozotocin, D-pinitol has been demonstrated to increase the insulin sensitivity of insulin target tissues. [
7,
8,
9]. Studies have shown that insulin secretion increases in diabetic animal models following administration of ice plant extract (IPE) and its active compound, D-pinitol [
6,
8,
10,
11,
12]. However, the mechanisms by which IPE and D-pinitol can affect glucose-stimulated insulin secretion (GSIS) in vitro have not yet been investigated. Therefore, the present study was designed to evaluate the effectiveness of IPE and D-pinitol on glucose-stimulated insulin secretion (GSIS) in vitro.
Diabetes is a group of metabolic diseases characterized by high blood glucose levels. It occurs when the body cannot properly produce or use insulin, a hormone released by pancreatic cells in response to changes in plasma glucose levels. There are two main types of diabetes. Type 1 diabetes is an autoimmune disorder whereby the body’s immune system attacks and destroys insulin-producing beta cells in the pancreas. Type 2 diabetes (T2D) occurs when the body becomes resistant to insulin or when the pancreas cannot produce enough insulin to maintain normal blood glucose levels [
13]. T2D is mostly treated with insulin, oral medications, and lifestyle modifications. The goal of the drugs currently used to treat diabetes is to enhance the amount of insulin released by pancreatic cells, improve the insulin sensitivity of insulin target tissues, or both [
14]. Available medications for T2D include metformin, sulfonylureas, DPP-4 inhibitors, SGLT-2 inhibitors, and GLP-1 receptor agonists. Despite the development and application of insulin and the availability of contemporary anti-diabetic medications, unexpected side effects of already-existing drugs can significantly interfere with treatment, prompting researchers to explore more potent plant-based medications as an alternate course of treatment for diabetes [
15,
16].
Insulin secretion was found to be impaired following glucose injection by the homozygous disruption of pancreatic and duodenal homeobox-1 (PDX-1) in a mouse model and in human patients. PDX-1 is an essential insulin transcription factor that contributes to insulin secretion [
17]. In addition, for the regulation of pancreatic cell neogenesis and differentiation, PDX-1 is essential [
18]. Accumulated evidence has identified PDX-1 as a potential regulator of insulin secretion. To the best of our knowledge, no prior studies have reported on factors impacting the PDX-1 pathway.
This work is the first to clarify the molecular mechanism via which ice plant’s primary active ingredient, D-pinitol, increases insulin secretion in pancreatic cells, mainly through its involvement in the PDX-1 pathway. Additionally, based on the physiological effects of D-pinitol, this study assessed the glucose-reducing effect of ice plant in diabetic rats.
2. Materials and Methods
2.1. Plant Material
Ice plant (M. crystallinum) was acquired from JS Farmer Co., Ltd. (Jinju, Republic of Korea), and identified by DOWGENE Co., Ltd. (Seoul, Republic of Korea). The leaves and stems of M. crystallinum were collected on 24 February, then washed and dried.
2.2. Preparation of M. crystallinum
The ice plant extract was manufactured by S&D Co., Ltd. (Cheongju, Republic of Korea). M. crystallinum was extracted with ethanol, and the extract was concentrated. After mixing in dextrin relative to the solid content of the concentrate, the concentrate was dried to manufacture ice plant extract (IPE).
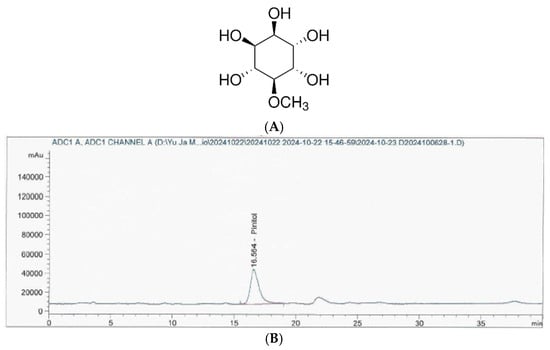
2.3. Analysis of D-Pinitol in IPE
The Korea Health Functional Food Association (Seongnam, Republic of Korea) analyzed the D-pinitol content in the IPE (
Figure 1). Briefly, HPLC analyses were conducted using an Agilent 1260 Infinity pumping system (Santa Clara, CA, USA) equipped with an Alltech 3300 evaporative light-scattering detector (ELSD, Milwaukee, WI, USA) and a YMC-Pack Polyamine II column (25 cm × 4.6 mm, 5 µm (YMC, Kyoto, Japan), flow rate 1.0 mL/min).
2.4. Cell Culture
RPMI-1640 medium (Cellgro, Manassas, VA, USA) was used to grow INS-1 cells (Biohermes, Shanghai, China), a rat insulin-secreting β-cell line. The composition of the cell culture medium and cell culture conditions were based on a previous study [
19].
2.5. Cell Viability Assay
To ascertain the non-toxic concentration ranges of IPE and D-pinitol for a 24 h period, INS-1 cells seeded in 96-well plates were assessed using an Ez-Cytox cell viability detection kit (Daeil Lab Service Co., Seoul, Republic of Korea). Using a microplate reader (PowerWaveXS; Bio-Tek Instruments, Winooski, VT, USA), the absorbance of the culture media of INS-1 cells treated for 24 h in the presence or absence of IPE and D-pinitol was determined at 450 nm.
2.6. GSIS Assay
A GSIS assay examined insulin secretion in INS-1 cells treated with IPE and D-pinitol for one hour or left untreated in Krebs–Ringer bicarbonate HEPES buffer (KRBB). The composition of KRBB was based on previous research [
20]. The basal and stimulant glucose concentrations were 2.8 and 16.7 mM, respectively. The KRBB of the untreated and treated cells was obtained by centrifugation, and GSIS was determined using a rat insulin ELISA kit (Gentaur, Shibayagi Co., Ltd., Shibukawa, Gunma, Japan), following the manufacturer’s instructions. The glucose stimulation index (GSI) was determined as the ratio of the insulin level at the basal glucose concentration to the insulin level at the stimulating glucose concentration.
2.7. Western Blot Analysis
Total protein was extracted using a radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA, USA). A sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 10%) gel was used to separate equal amounts (30 μg/lane) of total cellular protein, which were then transferred onto a nitrocellulose membrane (BioRad, Hercules, CA, USA). Using primary antibodies against P-IRS-2 (Ser731), IRS-2, P-IRS-1, IRS-1, P-PI3K, PI3K, P-Akt (Ser473), Akt, PDX-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling), polyvinylidene fluoride (PVDF) membranes were incubated at 4 °C overnight, and then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies (Cell Signaling Technology) and with an enhanced chemiluminescence reagent (GE Healthcare UK Limited, Buckinghamshire, UK) for protein visualization using a chemiluminescence system (FUSION Solo, PEQLAB Biotechnologie GmbH, Erlangen, Germany).
2.8. Animal Care and Diabetes Induction
Male Sprague Dawley rats (five weeks old) were purchased from BioLink and acclimated to the housing facility for one week, with free access to food and water. The rats were fed a high-fat diet (HFD, D12492, 60% kcal from fat; Research Diet Inc., New Brunswick, NJ, USA). After one week, diabetes was induced by intraperitoneal injection of streptozotocin (STZ; Sigma, Livonia, MI, USA, 30 mg/kg in 0.1 M citric acid buffer, pH 4.5) at a dose of 1 mL/kg, administered twice with a one-week interval. Following induction, the rats were grouped according to their blood glucose levels measured from tail vein samples, with 8 rats per group. Six treatment groups (comprising 8 individuals each) were used: (1) normal diet control group (ND): ND and administration of sodium carboxyl methyl cellulose (CMC) solution; (2) HFD control group: HFD after administration of the CMC solution; (3) positive control group: HFD and administration of metformin (MT) at 250 mg/kg; (4) experimental group: HFD and administration of IPE at 100 mg/kg; (5) experimental group: HFD and administration of IPE at 200 mg/kg; and (6) experimental group: HFD and administration of IPE at 400 mg/kg. All treatment compounds were dissolved in CMC solution and administered orally, once daily, for 9 weeks. Body weight was monitored weekly throughout the study. At the end of the experiment, the animals were euthanized, and epididymal fat was collected for weight measurements. This study was approved by the Institutional Animal Care and Use Committee of Gachon University (GU1-2024-IA0027-00, approved on 15 July 2024).
2.9. Oral Glucose Tolerance Test (OGTT)
Oral glucose tolerance tests (OGTTs) was performed in the 9th week after diabetes induction. Before testing, the rats were fasted for 16 h, and glucose (D-(+)-Glucose, Sigma, USA) was administered orally at 2 g/kg. Blood samples were collected from the tail vein at 0, 30, 60, and 120 min, and glucose levels were measured using a blood glucose monitoring system (Accu Check, Mannheim, Germany). The area under the curve (AUC) for the OGTT was calculated using the GraphPad Prism 5.00 software.
2.10. Biochemical Assay
The animals were euthanized after fasting for 12 h, and blood samples were collected from the abdominal aorta. The samples were centrifuged at 2000× g for 15 min at 4 °C to obtain serum stored at −70 °C for further analysis. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride (TG), total cholesterol (TC), and glucose levels were measured at a specialized testing facility (GENIA, Seongnam, Republic of Korea). Insulin levels were determined using a rat insulin ELISA kit, following the manufacturer’s instructions.
2.11. Statistical Analysis
Statistical significance was determined using one-way analysis of variance (ANOVA) and multiple comparisons with Bonferroni correction. p was set at p < 0.05, indicating statistical significance. All the analyses were performed using SPSS Statistics ver. 19.0 (SPSS Inc., Chicago, IL, USA).
4. Discussion
Among the several ingredients of IPE, one of the primary active components responsible for the biological actions of IPE is D-pinitol [
21]. D-pinitol has been shown to have various anti-inflammatory, anti-cancer, and antioxidant qualities; such qualities have been extensively researched in the context of diabetes [
6,
8]. However, the mechanisms by which IPE and D-pinitol affect GSIS in vitro are yet unknown. The effect of IPE and D-pinitol on insulin secretion in vitro was examined in this study, along with the possibility that the PDX-1 pathway plays a role in the insulin secretion action of these substances.
Previous studies have demonstrated the positive impact of IPE and D-pinitol on insulin secretion in diabetic animal models [
6,
8,
10,
11,
12]. Consistently with previous studies, we found that treatment with IPE and D-pinitol increased insulin secretion in INS-1 cells. In contrast, it was reported that D-pinitol from
Ceratonia siliqua decreased GSIS in INS-1 cells [
22]. The differences noted in our study may be due to the variation in exposure times to glucose and D-pinitol, because insulin-secreting β-cells may change their function when exposed to high glucose concentrations for an extended period of time [
23]. Research on how D-pinitol affects insulin secretion has produced conflicting findings. Only a few studies have demonstrated that D-pinitol decreases insulin secretion, whereas most investigations have shown that it increases insulin secretion in various experimental models. Similar findings were observed in the present study [
6,
8,
10,
11,
12].
We performed mechanistic experiments using Western blotting to examine the potential mechanisms underlying the in vitro effects of IPE and D-pinitol on GSIS. Artificially or spontaneously produced reduced expression of PDX-1 in pancreatic cell lines affects GSIS, suggesting that PDX-1 functions as a gene required for GSIS [
17]. Given the significance of PDX-1 in islet formation and insulin gene transcription, it is plausible that insulin gene transcription is decreased by PDX-1 deficiency, which in turn reduces insulin production [
24]. Therefore, we examined whether this was related to the effects of IPE and D-pinitol on INS-1 cells. In cells following IPE and D-pinitol treatment, PDX-1 expression was much higher than in control cells in this study.
Based on our observations, PDX-1 could be directly or indirectly targeted by D-pinitol or IPE. An upstream PI3K/Akt signaling pathway mediates PDX-1 [
25]. A previous study indicated that increased insulin secretion is the outcome of activating PI3K/Akt signaling, which controls the postnatal proliferation and size of individual pancreatic β-cells. PI3K/Akt signaling is facilitated by IRS-2, an upstream activator [
26]. Thus, the overexpression of PDX-1 via the IRS-2/PI3K/Akt pathway could upregulate insulin secretion, which might be one of the primary insulin secretion mechanisms. Consistently with prior research, this study found that following IPE and D-pinitol treatment, there was a significant increase in the expression of IRS-2, PI3K, and Akt compared to control cells. However, further research is required to fully understand how IPE and D-pinitol regulate the expression of PDX-1 via its upstream activators, including IRS-2, PI3K, and Akt. Therefore, it is conceivable that IPE and D-pinitol attenuate hyperglucagonemia by increasing insulin secretion, partially through the overexpression of PDX-1 via the IRS-2/PI3K/Akt pathway. These findings suggest that diabetes can be effectively treated with these benefits of IPE and D-pinitol, as there is a lower chance of hypoglycemia.
An HFD induces adiposity and insulin resistance, both of which are prominent features of T2D [
25]. In the present study, IPE administration mitigated the excessive weight gain observed in the diabetic models, potentially through improved metabolic regulation. These findings align with previous studies demonstrating the efficacy of certain plant extracts in reducing weight and improving metabolic profiles [
26]. However, we found that weight gain was reduced only marginally at certain doses of IPE. Therefore, it is not clear from this study whether the weight improvement was due to the glucose-lowering effect of IPE. From a scientific standpoint, previous research has shown that T2D is recognized to be associated with obesity, but there is little information on how weight changes relate to the risk of diabetes [
27]. The results of the OGTTs showed a notable improvement in glucose tolerance in the IPE-treated HFD/STZ models, as evidenced by a reduction in the OGTT-AUC. This suggests that IPE enhances insulin signaling and glucose metabolism, likely by modulating pathways associated with insulin sensitivity [
28]. Furthermore, IPE treatment significantly reduced serum ALT, AST, triglyceride, and total cholesterol levels, highlighting its liver-function-ameliorating and lipid-lowering properties. These results suggest that IPE may mitigate the metabolic complications associated with diabetes [
29]. One of the critical mechanisms underlying insulin resistance is the impairment of insulin signaling via IRS-1 [
30]. IPE treatment increased IRS-1 phosphorylation, indicating the restoration of insulin signaling. This restoration is essential to improve glucose metabolism and insulin sensitivity. These findings support the idea that plant-based extracts can regulate and enhance metabolic functions by modulating key signaling pathways [
31,
32].
Despite the beneficial effects and mechanisms that we observed, our study had some limitations. This study used the HFD/STZ model, but did not fully replicate all the characteristics of T2D, indicating the need for further animal model studies. In addition, the molecular mechanisms underlying the insulin-secretion-promoting effects of IPE and D-pinitol have not been fully elucidated and require further investigation. In pancreatic cells, glucose transporter 2 plays a significant role through the adenosine triphosphate (ATP)-sensitive, potassium channels-dependent, classical insulin secretion route. The metabolism of glucose increases the ATP/adenosine diphosphate (ADP) ratio within β-cells. Higher ATP levels cause ATP-sensitive potassium channels to close. Therefore, future studies should further explore the potential mechanisms underlying the insulin secretagogue effects of IPE and D-pinitol. The lack of animal and clinical data is a limitation of our study, but it provides an important basis for future research.
5. Conclusions
The effects of IPE and one of its active compounds, D-pinitol, on insulin secretion are mediated by the upregulation of IRS-2, PI3K, Akt, and PDX-1 expression. More scientific investigation is required to support the beneficial use of D-pinitol as a novel anti-diabetic molecule. Additionally, we demonstrated its ability to reduce weight gain, improve glucose tolerance, and regulate serum lipid levels. Henceforth, the anti-diabetic action of IPE is partially verified by our scientific evidence, confirming that its efficiency is due to its active compounds, such as D-pinitol.