1. Introduction
Boron carbide is one of the two known types of diamond-like carbides, and it is characterised by its high hardness. Its properties make it useful for applications such as abrasives for polishing, ballistic armour, and neutron radiation absorption [
1]. One of the most interesting applications of boron carbide in medicine is its use as a potential boron carrier for boron neutron capture therapy (BNCT) [
2,
3]. BNCT is a treatment modality aimed at improving the therapeutic ratio for traditionally difficult to treat tumours. It is based on nuclear capture and fission following the irradiation of nonradioactive boron-10 with low thermal neutrons. The most challenging aspect of successful treatment with BNCT is the precise delivery of boronated compounds to the tumour [
4].
The primary advantage of boron carbide (B
4C) is its high boron content (80–91 at.%) [
5]. These results show that synthesised boron carbide has got a significant concentration of the desirable
10B isotope. In addition, this covalent compound is chemically inert and degrades slowly in the body. Previous boron carriers for BNCT therapy, such as BPA (o-boronophenylalanine) and BSH (sodium salt of thioborate anion), have low concentrations of the desired element. The surface of the obtained boron carbide was modified with compounds containing functional groups to enhance the selectivity and efficiency of the carrier. Functional groups enable the attachment of various trope molecules to the surfaces of nanoparticles, which will selectively deliver boron to the tumour environment.
Dextrin and dextran T70 contain a carboxyl group (COOH) in their structure, whereas lysine and arginine amino acids possess both a carboxyl group (COOH) and an amine group (NH2). In contrast, sorbitol is characterised by numerous hydroxyl (OH) groups. These functional groups provide a means of attaching specific proteins that can interact with the targeted cancer cells.
In large-scale production, boron carbide is typically derived from boric acid or boron oxide via carbothermic reduction [
6]. This process entails heating the acid or oxide with carbon to 2000 °C, utilising various forms of carbon such as graphite, carbon nanotubes, and carbon black. However, this technique has drawbacks, as it results in a coarse-grained product with a high concentration of impurities, as well as the loss of boron due to the oxidation of boron oxide, leading to a heterogeneous end product [
7,
8]. An alternative method is magnetothermal reduction, which involves the reduction of boron anhydride in the presence of carbon, magnesium, or its alloys. This process must be carried out under protective gases, such as argon or hydrogen, owing to the high vapour pressure of magnesium. The resulting powder is fine, as the magnesium oxide present in the system inhibits the growth of boron carbide grains. However, this method also has disadvantages, because stable magnesium borides are present in the final product [
9,
10,
11].
A method for obtaining boron carbide for special applications, such as the production of B
4C with a high boron content, is the elemental synthesis method, which utilises high-purity elements to guarantee a product that is free of impurities [
8]. The process of obtaining boron carbide using this method involves two steps. First, a stoichiometric mixture of amorphous boron and carbon is granulated and then placed in an inert atmosphere or vacuum at 1500 °C.
In the realm of boron neutron capture therapy (BNCT), the use of traditional boron compounds is limited by therapeutic efficacy, primarily because of the short duration of their concentration in tumour cells. To enhance the efficacy of this treatment, researchers have sought to optimise the delivery of boron compounds to the tumour tissues, thereby increasing the boron concentration and the contrast in boron content between tumour and normal tissues. One approach to achieve this goal involves the functionalisation of boron carbide nanoparticles with reactive functional groups, such as –COOH or –NH
2, which may facilitate the attachment of a dye or peptide. In a recent study, commercial boron carbide was functionalised using the fluorescent dyes triethylalanine and lysamine, resulting in the attachment of reactive amine groups. The modified nanoparticles were shown to translocate into mice with malignant skin tumour and thymoma cells, inhibiting the growth of neighbouring cells after neutron irradiation. While this approach improves therapeutic efficacy, it also leads to increased particle agglomeration, potentially impeding the penetration of the carrier into tumour cells [
12,
13,
14].
An alternative method for modifying boron carbide surfaces to improve therapeutic efficacy involves coating B
4C particles with positively charged poly-L-lysine and negatively charged polyglutamic acid followed by conjugation with transferring. In vitro tests on the cervical cancer cell line HeLa and healthy cells derived from rat kidney NRK demonstrated that the modified B
4C was taken up by cancer cells via the transferrin receptor, but not by the NRK cells. Furthermore, when the conjugate was injected intravenously into mice, it was transported to the tumour and internalised by the cancer cells near the nucleus via endocytosis. These findings suggest that the modified B
4C conjugate is promising as a potential boron delivery agent for BNCT [
15].
In the next approach, using a pulsed laser irradiation method, boron carbide particles approximately 200 nm in size were obtained. The obtained particles were encapsulated in a graphite layer. This modification makes it possible to obtain particles characterised by easier conjugation with a given anticancer drug or to enable a specific tissue recognition ability. The graphite-coated B
4C modified in this way was tested in vitro to investigate the utility of boron neutron capture therapy in a human squamous cell carcinoma (SCC) cell line and in nude SAS heterotransplanted mice. In this model, boron carbide particles are directly injected into tumours [
16].
The primary objective of this study was to optimise the surfaces of boron carbide nanoparticles through modification with compounds such as dextrin, dextran T70, sorbitol, lysine, and arginine. Surface modification in subsequent stages of the research will facilitate the functionalisation of nanoparticles to target the tumour environment.
2. Materials and Methods
2.1. Synthesis of Boron Carbide
In a graphite crucible, a layer of amorphous boron was placed on a graphite pad and a layer of carbon in the form of amorphous carbon black was placed on top, maintaining a molar ratio of 4:1. The mixed powders were subjected to a heat treatment process at 1650 °C in an argon stream for 2 h. After synthesis, the resulting powder was subjected to a grinding process in a rotary vibrating mill for 24 h in isopropanol.
2.2. Preparation of Boron Carbide Suspension
The powder obtained by synthesis and grinding was chemically digested using alternating concentrated HCl and concentrated HNO3 solutions. The powder was then washed repeatedly with distilled water to obtain a pH suspension of 6.5–7.5, which was then centrifuged at the final stage. Distilled water was added to the resulting suspension to obtain the desired concentration of boron carbide nanoparticles. It was then exposed to UV light, centrifuged, and filtered through a biological filter. This process was repeated until the pH of the suspension was 6.8–7.2.
2.3. Functionalisation of Boron Carbide Surfaces
To prepare the polysaccharide and amino acid solutions, a sodium chloride solution was prepared at an ionic strength of 0.01 M. Appropriate amounts of dextrin, dextran T50, dextran T70, sorbitol, lysine, and arginine were dissolved successively in the NaCl solution thus prepared. For sugar and its derivatives, the concentration of functionalised compounds ranged from 0 to 400 mg, while for amino acids it ranged from 0 to 20 mg. Samples (5 mL) were then prepared by mixing the boron carbide and modifier suspensions in a 1:1 volume ratio. The samples were subjected to a shaking process for 3–7 days.
2.4. Particle Size Distribution
The diffusion coefficients of bare boron carbide and boron carbide with polysaccharides and amino acid complexes were determined by dynamic light scattering (DLS), which provided the hydrodynamic diameter the nanoparticles. Particle size was determined using a Zetasizer Nano series instrument (Melvern Instruments, Malvern, UK).
2.5. Zeta Potential
The electrophoretic mobility of bare boron carbide and boron carbide with polysaccharides and amino acid complexes were determined by the Laser Doppler Velocimetry (LDV) technique, also using the Zetasizer Nano instrument from Malvern. The zeta potentials were calculated using the Smoluchowski model. The dependence of the zeta potential change on the modifier concentration allowed us to determine the optimum concentration for the functionalisation of the boron carbide surface for bioassays.
2.6. FTIR Spectroscopy and NMR
The structure of the samples dried at 60 °C was studied by transmission spectroscopy. Measurements were performed with a Bruker Vertex 70v (Bruker, Billerica, MA, USA) spectrometer using the standard KBr Lozenge method. The measurement consisted of 128 scans in the range of 4000–400 cm−1, recorded at a resolution of 4 cm−1. The pure modifiers were also examined. High-resolution, solid-state 11B MAS-NMR spectra were measured using the APOLLO console (Tecmag, Houston, TX, USA) and a 7 T, 89 mm superconducting magnet (Magnex) (Tecmag Inc., Houston, TX, USA).
2.7. Inductively Coupled Plasma Optical Emission Spectrometry
The concentration of boron in the prepared suspensions B
4C (fraction II with a concentration of 1208 mg/L) and B
13C
2 (fraction I with a concentration of 4471 mg/L) was analysed using the technique of inductively coupled plasma optical emission spectrometry —ICP-OES—in accordance with ISO 11885 (2007) [
17], on a Perkin Elmer Optima 7300DV instrument (Waltham, MA, USA). During the study, a calibration curve was prepared based on five points (including a blank sample), covering concentrations from 0.05 to 10 mg/L. Both of the certified boron reference materials used for the calibration curve and laboratory control sample to control the calibration curve were produced according to ISO 17034 (2016) [
18], i.e., Boron B—10 g/L in H
2O for ICP (CPAchem Ltd., Bogomilovo, Bulgaria) and ICP multi-element standard solution IV (Supelco
® Analytical Products, Sigma-Aldrich, St. Louis, MO, USA). The analyses were conducted by observing the boron emission spectrum at a wavelength of 249.770 nm. Before analysis, the boron carbide suspension was thoroughly shaken on a vortex laboratory mixer, then diluted 500 times by taking 20 µL of the suspension, which was quantitatively transferred to a 10 mL volumetric flask. The suspended material was dissolved in 1 mL of concentrated nitric acid (V) (Nitric acid 67%, NORMATOM
® (VWR, West Chester, PA, USA) for trace metal analysis), and then topped up to 10 mL with deionised water obtained from the Direct-Q 3 UV system (Merck Millipore, Burlington, MA, USA). The obtained solutions were then directly analysed using the ICP-OES technique.
2.8. Preparation of Bioassay Samples
Appropriate amounts of dextrin, dextran T70, dextran T50 sorbitol, arginine, and lysine were dissolved in the prepared NaCl solution at an ionic strength of 0.02 M so that when mixed with a 2000 mg/L boron carbide suspension at a volume ratio of 1:1, they gave concentrations of 100 mg/mL for dextrin and dextran T70 and 2 mg/mL for sorbitol, arginine, and lysine. These mixtures were then placed on a shaker for seven days to intensify the mixing and functionalise the surface.
2.9. Cell Culture
T98G human glioblastoma multiforme cells obtained from the American Type Culture Collection (ATCC; CRL-1690) were used for this study. The cells were cultured in Eagle’s Minimum Essential Medium (EMEM; ATCC) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal bovine serum (FBS; all from Sigma-Aldrich) at 37 °C with 5% CO2 and 95% humidity.
The T98G cells were seeded in 96-well plates (0.5 × 104 cells/well). After 24 h, native boron carbide and boron carbide functionalised with dextran T70, dextrin, arginine, lysine, and sorbitol were added at concentrations ranging from 0.1 μg/mL to 400 μg/mL for 24, 48, and 72 h. MTT dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 5 mg/mL) (Sigma-Aldrich) was added after incubation for 4 h and then lysed overnight in lysis buffer (N,N-dimethylmethanamide, sodium dodecyl sulfate, and water) at 37 °C. Absorbance was measured at 570 nm using a Thermo Labsystems Multiskan RC microplate reader (Thermo Fisher Scientific Inc., Waltham, MA, USA) with Genesis Lite 3.05 software (Thermo Life Sciences).
3. Results
This section presents a detailed analysis of the physicochemical and biological properties of boron carbide (B4C) particles and their surface modifications with various compounds. The experiments aimed to evaluate the impact of modifiers on key parameters such as zeta potential, particle size distribution, and surface chemistry, as well as their cytotoxicity against glioblastoma T98G cells. Through a combination of electrophoretic mobility, FTIR, NMR, and ICP-OES techniques, we assessed the interaction dynamics between B4C and the chosen modifiers. These insights pave the way for understanding the stability, functionality, and potential biomedical applications of modified B4C nanoparticles.
The discussion begins with an investigation into zeta potential, which provides critical information about the surface charge changes resulting from adsorption processes.
3.1. Zeta Potential
The adsorption of seven different modifiers (dextrin, dextran T50, T70, arginine, lysine, sorbitol) was studied by monitoring changes in the electrophoretic mobility (zeta potential) of boron carbide particles induced by this process. The experimental procedure consisted of following steps:
- –
The zeta potential of bare boron carbide particles in suspension with a concentration equal to 100 mg L−1 was measured,
- –
Modifier covered boron carbide particles were formed in situ by filling the cell with the modifier suspension of an appropriate concentration for 10 min. After that, the electrophoretic mobility of the new complex was measured using electrophoretic methods.
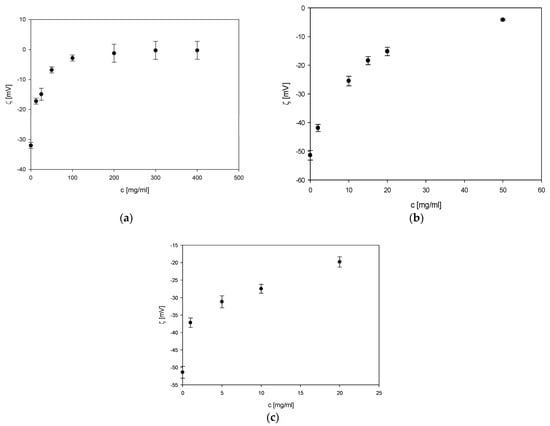
As can be seen in
Figure 1a–c, in all cases, the zeta potential of boron carbide increases monotonically with the modifier concentration. The zeta potential value varied considerably up to a certain point, at which point the value stabilised.
Figure 2a–c shows the zeta potential changes for modifiers, such as sorbitol, arginine, and lysine. For sorbitol and amino acids, slight changes in the zeta potential of the formed complex were observed. However, low zeta potential values suggest the formation of a more stable suspension.
3.2. Particle Size Distribution
Figure 3a–c shows the results of the hydrodynamic diameter depending on the concentration of the modifiers, which were dextrin, T50 dextran, and T70 dextran, respectively. As the concentration increased, there were clear changes in the particle size: the average diameter increased.
Figure 4a–c shows the results of the hydrodynamic diameter depending on the concentration of the modifiers, which were sorbitol, arginine, and lysine, respectively. The changes in the mean diameter are not as significant as in the case of the previous modifiers, and this is also influenced by the concentration at which the boron carbide surface was modified. This affected the size of the modified boron carbide particles, as little amino acid could attach to the surface of the boron carrier. Nevertheless, an increase in the diameter was noticeable at the lowest applied modifier concentrations. Ultimately, all of the compounds used as modifiers resulted in an increase in the particle size.
Based on measurements of electrophoretic mobility (zeta potential) and diffusion coefficient (hydrodynamic diameter), appropriate concentrations of all modifiers were chosen for the surface functionalisation of boron carbide. The following concentrations were selected: 100 mg/mL for dextrin and dextran T70 and 2 mg/mL for sorbitol, arginine, and lysine. Nanoparticles modified in a defined way were tested on the T98G glioma cells.
3.3. FTIR and NMR
Samples marked as B
4C and its modifications exhibited typical water bands at approx. 3400, 1640, and 600 (very broad band) cm
−1, as they were measured as a droplet on a KBr pellet. The FTIR measurements revealed that there were no additional bands present; therefore no chemical interaction occurred (
Figure 5). This is in line with the MAS-NMR results. This clearly shows that the interaction between the dextrin/lysin and ceramic particles is physical rather than chemical.
Analysis of the spectra indicates that boron carbide binds to the modifier in question, as evidenced by the appearance or disappearance of characteristic bands typical of pure boron carbide or the modifier.
As seen in
Figure 6, all of the tested samples exhibit exactly same NMR signals at approx. −25.4 ppm. This indicates that all of the tested materials possess the same B
4C bonds. This indicates that there is no reaction on boron’s side [
16,
19,
20,
21].
For the dextrin modification, no changes are visible in the NMR spectrum (
Figure 6) apart from the addition of a band at approximately 2 ppm indicating the presence of ceramics. The rest are typical for dextrin and spinning sidebands. In the case of lysine, the situation is similar, but an additional signal is visible at approximately 30 ppm, which also comes from lysine, but due to the lower S/N ratio was not as visible for the pure material. The MAS-NMR spectra do not indicate the presence of new chemical bonds in the material.
3.4. Inductively Coupled Plasma Optical Emission Spectrometry
ICP-OES analyses revealed that the concentration of boron present in suspension B4C—fraction I was 938 mg/L, which fairly closely matched the theoretical value of 945 mg/L boron. In the case of fraction 2, where the theoretical concentration of boron is 3819 mg/L, the analysis results indicated that the total boron concentration in this fraction was 385.8 mg/L.
3.5. Cytotoxic Activity of Functionalised B4C Compounds Against T98G Cells
The preparations named B
4C-dextrin and B
4C-sorbitol sedimented rapidly. The graphs show the results of three independent in vitro tests (for one test time point), in which each concentration of the analysed compound was tested in triplicate (
Figure 7,
Figure 8 and
Figure 9). Of all the compounds tested, B
4C-dextran T70 and B
4C-dextrin showed the highest toxicity to T98G cells, even after a short incubation time. For the other compounds, longer incubation times were required to observe full cytotoxicity (except for B
4C-sorbitol). The rapid sedimentation observed in the B
4C-dextrin and B
4C-sorbitol preparations indicates potential challenges in maintaining stable suspensions, which could impact their effectiveness and application in various contexts. The results from three independent in vitro tests, each conducted in triplicate for different concentrations, provide a robust dataset for evaluating the cytotoxic effects of the analysed compounds on T98G cells. These findings, presented in
Figure 7,
Figure 8 and
Figure 9, offer valuable insights into the relative toxicity of the tested substances.
Among the compounds examined, B4C-dextran T70 and B4C-dextrin demonstrated the most pronounced cytotoxic effects on T98G cells, even with brief exposure times. This rapid onset of toxicity suggests that these compounds may have potent cellular interactions or mechanisms of action. In contrast, the other tested compounds generally required extended incubation periods to elicit full cytotoxic responses, with B4C-sorbitol being a notable exception. These variations in cytotoxicity profiles and time-dependent effects highlight the importance of considering both compound composition and exposure duration when assessing potential biological impacts or therapeutic applications of these substances.
As can be seen in
Figure 10, in the TEM photos of compounds used for the functionalisation of boron carbide particles, we can notice an increased agglomeration of boron carbide particles when using different compounds for functionalisation, but in this method we were not able to determine the influence of the modifier on the surface of the particle.
4. Conclusions
In this study, the impact of different surface modifications of boron carbide on cytotoxicity against T98G glioblastoma cells was evaluated. The findings indicate that among the tested modifications, B4C-dextran T70 and B4C-dextrin exhibited the highest cytotoxicity. The analysis of zeta potential and particle size distribution confirmed successful functionalisation, while biological assays demonstrated that surface modifications significantly influence the interaction of nanoparticles with cancer cells.
It is important to emphasise that this study focused exclusively on negatively charged surface modifications. Although previous research suggests that surface charge is a key factor in tumour targeting, our results are strictly based on negatively charged samples. As a result, the influence of positively charged modifications was not assessed and remains an area for future research. Investigating positively charged modifications may provide further insights into the relationship between zeta potential and targeted tumour delivery, ultimately contributing to the optimisation of boron carbide-based nanoplatforms for BNCT applications.
These results mark an important step toward designing functionalised ceramic nanoparticles for biomedical applications, particularly in BNCT therapy.