1. Introduction
The production of hydrogen has recently garnered considerable attention due to its potential as a clean and sustainable energy source. As the global community strives to address the challenges posed by climate change and reduce reliance on fossil fuels, hydrogen emerges as a promising alternative [
1,
2]. Its versatility and high energy content, as well as our capacity to produce it from a range of renewable sources, underscore its significance in achieving a low-carbon future [
3].
Hydrogen generation can be achieved through various methods, including steam methane reforming [
4], electrolysis [
5], thermochemical water splitting [
6], biological processes [
7,
8], and photocatalytic water splitting [
9]. Each method presents unique advantages and challenges, with ongoing research focused on improving efficiency, reducing costs, and minimizing environmental impacts [
10]. Photocatalytic water splitting, in particular, offers a promising approach by using sunlight and semiconductor materials to directly produce hydrogen from water [
11].
Semiconductor nanomaterials are promising photocatalysts for hydrogen generation due to their unique properties, such as high surface area, efficient light absorption, suitable bandgap, and catalytic activity [
12]. These nanomaterials, such as TiO
2, ZnO, g-C
3N
4, CdS, and their binary [
13,
14] and ternary composites [
15,
16], are designed to absorb sunlight and facilitate the separation of water molecules into hydrogen and oxygen [
17]. Among them, cadmium sulfide (CdS) is of particular interest due to its optimal bandgap energy and visible-light absorption, making it a highly effective material for hydrogen production [
18,
19]. However, its efficiency is limited by rapid electron–hole recombination and photocorrosion. To address these issues, CdS can be decorated with metal sulfides such as ZnS, CuS, NiS, MoS
2, Bi
2S
3, etc. [
20]. This approach leverages synergistic effects between different semiconductors, such as improved charge-separation efficiency, expanded light-absorption range, and enhanced catalytic activity [
21]. Among the above-mentioned co-catalysts, Bi
2S
3 attracts particular interest due to its narrow bandgap, high absorption coefficient, and excellent photocatalytic properties [
22]. The incorporation of Bi
2S
3 into CdS nanoparticles not only improves the light absorption capacity but also facilitates better charge separation and transfer. This results in reduced recombination rates of photogenerated electron–hole pairs, significantly enhancing the photocatalytic efficiency [
23,
24]. Furthermore, CdS/Bi
2S
3 photocatalysts present notable advantages, including enhanced stability, elevated catalytic activity, adjustable band structure, and cost-effectiveness [
24,
25]. These attributes render them highly appealing for a spectrum of photocatalytic applications, spanning from environmental remediation to renewable energy generation [
26]. However, the CdS/Bi
2S
3 system has notable limitations, including photocorrosion, poor stability, rapid charge recombination, and inefficient charge transfer. Other challenges involve scalability issues, limited light absorption, Bi
2S
3 degradation, and suboptimal photoelectrochemical performance, all hindering practical applicability [
27]. Addressing the limitations of the CdS/Bi
2S
3 system involves surface modification for stability, heterojunction engineering and co-catalyst decoration for improved charge separation, cadmium substitution to reduce toxicity, bandgap tuning for broader absorption, optimized synthesis for scalability, and nanostructuring to enhance photoelectrochemical performance [
27,
28].
The literature contains several papers demonstrating that CdS/Bi
2S
3 can generate hydrogen through water splitting. To illustrate this point, in the work [
29], Yang et al. synthesized 2D Bi
2S
3/CdS nanosheet arrays through a three-step complex process and utilized them as photoanodes, achieving improved photoelectrochemical hydrogen evolution. Another CdS/Bi
2S
3 was prepared via a one-step solvothermal method and exhibited an exceptional photocatalytic hydrogen production performance [
30]. Lately, binary CdS/Bi
2S
3 heterostructures were fabricated using ZnO/Bi
2S
3 as active intermediates. The highest photocatalytic hydrogen evolution reaction rate was 3.85 mmol∙g
−1∙h
−1 [
31].
In addition to the abovementioned approaches, CdS/Bi
2S
3 nanocomposites can be synthesized also through techniques such as sonochemistry [
23], hydrothermal [
32], wet-chemistry approach [
33], ion exchange [
34,
35], one-pot controlled synthesis [
36], in situ fabrication [
37], sol–gel [
38], the SILAR method [
39], chemical-bath deposition [
40], and mechanochemical [
41,
42] in order to be employed in various applications. Nevertheless, the prevailing synthesis techniques, including sol–gel, hydrothermal, and chemical-bath deposition, are encumbered by considerable limitations [
43]. These include the utilization of toxic solvents, intricate reaction conditions, and scalability challenges. In contrast, mechanochemical synthesis offers a solvent-free, environmentally friendly, and scalable alternative [
44,
45]. By employing mechanical energy to propel the reaction, our methodology obviates the necessity for deleterious solvents, streamlines the process, and permits synthesis under ambient conditions [
42]. Moreover, the ball-milling process fosters superior contact between CdS and Bi
2S
3, thereby enhancing charge separation and augmenting photocatalytic efficacy.
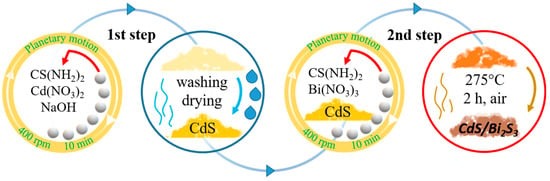
This study aims to develop a cost-effective, solvent-free, and scalable method for synthesizing Bi2S3-decorated CdS nanoparticles with enhanced photocatalytic hydrogen generation capabilities. We hypothesize that the mechanochemical approach, in conjunction with thermal annealing, will improve charge separation and minimize electron–hole recombination, thereby enhancing hydrogen-evolution performance. To the best of our knowledge, this is the first time that the mechanochemical route is applied to the solid-state production of CdS/Bi2S3 nanocomposites.
4. Conclusions
In situ solid-state synthesis of CdS/Bi2S3 nanocomposites via the ball-milling technique, with further thermal annealing, was successfully performed in this study. The morphology, structure, and composition of the individual CdS and Bi2S3 samples, as well as the composite CdS/Bi2S3, were thoroughly characterized using XRD, Raman spectroscopy, FTIR, SEM, EDS, and TEM techniques. The XRD analysis and Rietveld refinement revealed that both the CdS and composite samples contain cubic and hexagonal phases of CdS. The bandgap energies were found to be 2.4 eV for CdS, 1.3 eV for Bi2S3, and 2.31 eV for the CdS/Bi2S3 composite. Moreover, synthesized samples were tested for their ability to perform photocatalytic hydrogen evolution. The highest HER was observed in the CdS/Bi2S3/Pt sample, reaching 996.68 μmol h−1g−1 (AQE 0.87%) at 3.5 h of solar-light irradiation. In contrast, the CdS/Pt sample reached its peak HER at the 4th hour, with a value of 748.42 μmol h−1g−1 (AQE 0.67%). The Bi2S3/Pt sample did not produce any H2. Thus, the obtained results showed that combining CdS, Bi2S3, and a Pt co-catalyst produced a synergistic effect, increasing the HER by 1.3 times when comparing CdS/Bi2S3/Pt to CdS/Pt. The nanocomposite exhibits a strong photocatalytic hydrogen-generation performance with various sacrificial agents and retains significant stability over repeated use. These findings highlight the material’s potential for sustainable hydrogen production in energy applications.