1. Introduction
Plaque, also referred to as calculus, is a biofilm composed of bacteria and other organisms that forms during the chewing of food. This biofilm is made up of a diverse array of microorganisms that are densely packed together and stabilized by a network of organic polymers produced by both bacterial activity and saliva [1,2,3]. Similar to biofilms found in various environments, dental plaque forms on the surfaces of teeth in the oral cavity. When bacteria aggregate and adhere to a surface, a biofilm is created. These bacteria synthesize an extracellular polymeric matrix that encapsulates them [4]. Research shows that this extracellular polysaccharide matrix is synthesized by bacterial species such as Staphylococcus sp., Streptococcus sp., Bacillus sp., Enterobacter sp., Corynebacterium sp., Micrococcus sp., and Klebsiella sp. [3,5]. Bacillus sp. is commonly present in the oral cavity. These species are typically considered transient in a healthy individual’s mouth. Initially nonpathogenic, they can become opportunistic and lead to various oral diseases [6,7].
Biofilm-forming bacteria differ from planktonic bacteria, which move freely in aquatic environments. Plaque biofilm bacteria can respond to various signals, including nutritional cues and cellular recognition of specific and non-specific surface attachment sites [8,9]. Biofilms can develop on a wide range of oral surfaces, such as enamel, dentin, gingiva, cementum, oral mucosa, carious lesions, restorations, dental implants, and dentures. The exposed root surface and coronal enamel surface are quickly colonized by plaque. Microbiota proliferate more rapidly on exposed root dentin surfaces than on enamel surfaces due to the irregular geometry of the former [10]. Surface-bound bacteria have a higher likelihood of survival and natural selection compared to their planktonic counterparts. Bacteria present in dental plaque exhibit greater resistance to antibiotic treatment than waterborne bacteria. The extracellular polysaccharides produced by plaque bacteria offer protection against various threats, including antibiotics, antibodies, surfactants, bacteriophages, and white blood cells [11]. Bacteria within a biofilm can also develop resistance to disinfectants. The minimum inhibitory concentration (MIC) of antimicrobial drugs is significantly higher (up to 1000-fold) for bacteria in biofilms compared to planktonic bacteria [12]. This condition is linked to tooth loss and poses a significant risk to global health [13,14].
In light of the growing prevalence of microorganisms that are resistant to conventional antibiotics, there is an immediate and pressing requirement for the development of additional effective treatments [15,16]. Clindamycin (CM) is an antibiotic that works by binding to the large subunit of the ribosome [17]. The mrsA and mefA genes encode efflux pump activity [18,19], while the linA gene is associated with O-nucleotidyltransferase activity [20], which is believed to inactivate CM. The prevention of plaque formation and maturation may be achievable through the discovery of new, strategic, and effective antibacterial agents [21,22]. Understanding the potential of metal nanoparticles to enhance the efficacy of antimicrobials against bacteria responsible for gum disease is crucial.
As a potential treatment method for conditions associated with biofilms, nanotechnology has demonstrated a great deal of promise. As vehicles for the administration of medication, nanoparticles offer a number of benefits, including a reduction in medication-related adverse effects and a prolonged release of the drug. The purported antibacterial properties of a number of nanoparticles have been the subject of a significant amount of research. Metallic oxide nanoparticles, such as zinc oxide nanoparticles (ZnO-NPs), exhibit low cytotoxicity and show significant potential as drug delivery systems. Research indicates that the antibacterial and antibiofilm properties of nanomaterials are strongly influenced by factors like their shape and surface charge [23,24,25,26]. ZnO-NPs provide several benefits, including improved solubility, bioavailability, and biocompatibility. They can interact with biomolecules, localize within organs, and contribute to cellular homeostasis [27]. ZnO-NPs also offer lower toxicity and cost compared to alternative nanomaterials, making them suitable for a wide range of applications, including cancer therapy, infection and inflammation prevention, and diabetes management [28,29]. Additionally, ZnO-NPs are characterized by their catalytic efficiency, chemical stability, and exceptional adsorption capability [30]. Therefore, this study aims to evaluate the antibacterial and antibiofilm properties of ZnO-NPs against oral Bacillus species and to assess the effectiveness of combining CM with ZnO-NPs in reducing antibiotic resistance.
3. Results and Discussion
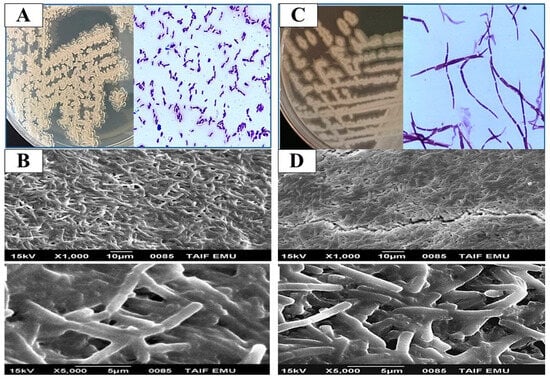
Bacillus species are part of the oral microbiota and play a key role in adapting to environmental changes within the protective biofilms that dominate the mucosal layer of the oral cavity [50,51]. While these microorganisms can persist as benign flora, they can also become opportunistic pathogens, leading to a variety of oral diseases. The microflora on the enamel are continuously adapting to environmental fluctuations within these biofilms [52]. Based on morphological and biochemical data, eight Bacillus isolates (16%) were identified from fifty isolates recovered from oral specimens. Our findings align with the morphological and biochemical characteristics typical of Bacillus species. The isolates were first confirmed at the genus level by assessing colony and cell morphology, catalase production, oxidase activity, motility, and Gram staining (Table 2). All isolates were identified as Gram positive, spore-forming, and motile rods (except the MD7 and MD8 strains, which were non-spore-forming and non-motile). All bacterial isolates tested positive for catalase production, except for the strain MD8. For the oxidase test, all isolates were oxidative, except for the H1 and H12 strains, which were characterized as non-oxidative bacteria (Table 2). To further investigate the biofilm-forming capabilities of representative strains, SEM was employed. Specifically, Bacillus licheniformis H1 and Bacillus thuringiensis H9 were examined (Figure 1). SEM images revealed that the bacterial cells were obstructed by extracellular secretions, and the extracellular matrix between the cells was also evaluated [3]. Several studies have demonstrated that oral cavity Bacillus species can be isolated [50,51,53].
To taxonomically identify the eight Bacillus species, the partial 16S rRNA gene sequence was used. Sequence homology was assessed using the BLAST tool on the NCBI website (http://www.ncbi.nlm.nih.gov/), comparing the 16S rRNA sequences with those of type strains available in the NCBI database (Table 3). Eight isolates were identified, MS-1, MS-2, MS-3, MS-4, MS-5, MS-6, MS-7, and MS-8, namely Bacillus licheniformis H1 (LN899788), Bacillus cereus H2 (LN899789), Bacillus thuringiensis H5 (LN899790), Bacillus thuringiensis H9 (LN899791), Bacillus subtilis H10 (LN899792), Bacillus subtilis H12 (LN899794), Bacillus megaterium MD7 (LN899809), and Bacillus amyloliquefaciens MD8 (LN899810), respectively. The phylogenetic relationship between the selected isolates and other related bacteria found in the GeneBank database is depicted in Figure 2.
Based on the MIC values of the ZnO-NPs, CM, and the combination of the ZnO-NPs and CM (Table 4) for each bacterial strain, their anti-biofilm formation activity was determined. As described above in the biofilm formation section, an estimate of biofilm formation was calculated. According to a microtiter dilution assay based on resazurin, all Bacillus strains were susceptible to the ZnO-NPs, with MIC values ranging from 1 to 64 µg/mL (Table 4). However, the CM-MIC values for the tested bacteria ranged from 2 to 128 µg/mL, with Bacillus megaterium MD7 showing the lowest MIC at 2 µg/mL. Compared to the ZnO-NPs or CM alone, the ZnO-NPs combined with CM exhibited superior antibacterial activity, with the MIC for the combination fluctuating between 0.25 and 16 µg/mL. This resulted in a 2–4-fold reduction in the MIC compared to the ZnO-NPs alone and a 4–8 fold reduction compared to CM alone (Table 4). The recombination of excitons in metal oxide nanoparticles may be hindered due to their small particle size and larger band gap. As a result, the increased availability of electrons leads to higher levels of reactive oxygen species (ROS), thereby enhancing the antibacterial properties of metal oxide nanoparticles [54]. The antibacterial efficiency of the ZnO-NPs was correlated with their ability to deform bacterial cells and induce ROS generation, as well as the release of Zn ions, leading to lipid peroxidation, protein oxidation, and DNA damage in bacterial cells [55]. The electrostatic interaction between the positively charged nanoparticles and negatively charged bacterial cells [56,57] was responsible for the suppression of bacterial growth and ROS production. The CM molecule contains reactive groups such as hydroxyl and amide that readily chelate with ZnO-NPs [55].
The interaction between CM and the ZnO-NPs resulted in synergy, as indicated by the FIC analysis of the ZnO-NPs-CM combination, shown in Table 5. The combination was synergistic for most Bacillus species tested, with FIC values of 0.375 for B. licheniformis H1 and B. cereus H2, 0.47 for B. thuringiensis H9, and 0.5 for B. subtilis H10, B. megaterium MD7, and B. amyloliquefaciens MD8. Only two strains, B. thuringiensis H9 and B. subtilis H12, exhibited additive interactions (Table 5). Due to its low MIC values, the solution containing the ZnO-NPs and CM showed a higher synergistic effect against the evaluated clinical isolates. By combining the ZnO-NPs with CM and other medications, we aim to mitigate the detrimental effects of antibiotics on the host, enhance their bactericidal efficacy, and reduce the spread of antibiotic-resistant bacteria [58,59].
The viability of cancer cells (CAL27) and normal fibroblast cells (HFB4) after treatment with varying doses of the ZnO-NPs is shown in Figure 3. The ZnO-NPs exhibited stronger cytotoxicity against CAL27 compared to HFB4. The viability of CAL27 and HFB4 cells after treatment with 0.25–128 µg/mL ZnO-NPs ranged from 99.8% to 15.3% and 99.9% to 25.4%, respectively (Figure 3). These findings indicate that the ZnO-NPs had an IC50 of 52.15 µg/mL for CAL27 cells and 36.3 µg/mL for HFB4 cells. This supports the reliability and selectivity of the ZnO-NPs, which is consistent with the findings of Babayevska et al. [57] and Naiel et al. [56], who demonstrated that the ZnO-NPs are more destructive to cancer cells than to normal cells at comparable concentrations. These results align with previous studies [59,60]. The cytotoxicity induced by the ZnO-NPs in cancer cells is linked to the generation of ROS. Chakraborti et al. [61] argued that ROS production is primarily responsible for the anticancer effects of PEG-modified ZnO-NPs.
A significant concern with Bacillus species is their resistance to many antimicrobials. The ability of these bacteria to form biofilms is a major factor contributing to their resistance to antimicrobial treatments, making bacterial eradication more challenging [46]. This study quantified the biofilm-forming capacity of Bacillus strains using the MTP test (Supplementary Figure S1). Notably, 62.5% of Bacillus strains were significant biofilm producers (Table 6). B. licheniformis H1, B. thuringiensis H9, B. subtilis H10, B. megaterium MD7, and B. amyloliquefaciens MD8 were identified as strong biofilm producers, while B. cereus H2 and B. subtilis H12 exhibited only modest biofilm production. The B. thuringiensis H5 strain produced less biofilm than the other strains. These results may help explain the higher resistance rates observed in Bacillus strains to CM in this study.
When combined with the expansion of germs that are resistant to antibiotics, the failure to create new medications has resulted in an increase in the number of deaths and illnesses, particularly in healthcare settings [62]. To address this issue, novel strategies for preventing and eradicating bacterial biofilms are urgently needed. This study comprehensively evaluated the use of nanoparticle–antibiotic combinations to reduce bacterial biofilms (Table 6). The combination of the ZnO-NPs and CM showed significant antibiofilm activity against most strains, reducing biofilm formation from strong or moderate to non-producing, except for B. licheniformis H1 and B. thuringiensis H9, whose biofilm production capacity became weak. In contrast, the ZnO-NPs or CM alone had minimal impact on the adherence of Bacillus strains to biofilms (Table 6). Previous studies have also investigated the antibacterial and antibiofilm properties of ZnO-NPs against Bacillus strains [63].
The antibacterial and antibiofilm activities of ZnO-NPs are believed to result from the generation of ROS, such as superoxide anions, hydroxyl radicals, and hydrogen peroxide, which are harmful to bacterial cells [64]. The release of Zn2+ ions, resulting from the accumulation of ZnO-NPs in the outer membrane of bacterial cells, leads to membrane disintegration, protein degradation, and genomic instability, ultimately causing bacterial cell death. ZnO-NPs can also readily interact with antibiotic compounds that contain active groups, such as hydroxyl and amide groups, through chelation, significantly increasing their antimicrobial effectiveness. This enhances the diffusion and permeation of ZnO-NPs through biofilms [65]. Based on the findings of Ryan et al. [66], it has been demonstrated that ZnO-NPs possess the capability to disrupt the efflux pump system, which is an essential component of bacterial resistance to numerous antibiotics. Furthermore, ZnO-NPs encourage the creation of free radicals, which have a strong interaction with thiol-containing proteins in the bacterial cell wall. This interaction leads to an increase in the rate at which bacterial cells are damaged [67].
Table 6 provides an overview of the antibiotic resistance and biofilm production profiles of the strains investigated in this study. Except for B. thuringiensis H5 and B. subtilis H12, the expression of the ermC antibiotic resistance gene was prominently observed in the Bacillus strains. All strains carried the sipW biofilm gene, except for B. thuringiensis H5. Two strains also contained the tsaA gene, which is associated with biofilm production (Table 7). These findings indicate that B. licheniformis H1 and B. thuringiensis H9 expressed genes involved in both biofilm formation and antibiotic resistance. Overall, a strong correlation was observed between the presence of ermC genes and the biofilm genotype in most Bacillus strains. Caro-Astorga et al. [49] demonstrated the roles of several genes, including sipW and tasA, in biofilm formation. The tasA gene is particularly important, as its product is associated with the formation of amyloid-like fibers, which are responsible for the floating biofilms of Bacillus species. The identification of sipW is critical, as it encodes a protease involved in the metabolism of tasA. Furthermore, many studies have shown that erm genes contribute to bacterial CM resistance [68]. Methylation of the ribosomal target sites for CM and erythromycin confers resistance to these antibiotics in Bacillus species [69]. Cross-resistance to erythromycin, CM, and streptogramin B is mediated by the macrolide–lincosamide–streptogramin B (MLSB) resistance mechanism [70]. This is the most common mechanism of resistance to macrolides and lincosamides, primarily mediated by erm genes [71].
Source link
Maha A. Khalil www.mdpi.com