1. Introduction
The production of green hydrogen can be defined as a process which generates hydrogen based on some renewable source such as wind, solar, or biomass energy [
1]. In this way, green hydrogen can be considered a clean method of producing hydrogen which will emit less carbon dioxide than grey hydrogen, which is based on using fossil fuels [
1]. One promising method for producing green hydrogen is through electrolysis, where water is split to generate hydrogen and oxygen [
2]. In particular, this is an attractive option if the energy used to drive electrolysis is obtained from renewable sources. For example, Hosseini et al. consider electrolysis driven by either wind or solar power as a clean method for producing hydrogen [
3]. However, they also mention that one of the limitations of electrolysis is the high costs of the rare metals used in the electrodes [
3]. A comparison of the costs of producing hydrogen showed that green hydrogen combined with renewable energy might cost between 2.28 and 7.39
$/kg while grey hydrogen from methane or coal might cost 0.67–1.31
$/kg [
4]. Hence, there is a drive to make electrolysis systems cheaper and more efficient at producing hydrogen to make them more competitive. Discussion and comparisons of the different methods for producing hydrogen can also be found in the reviews of Acar and Dincer as well as Dash et al. [
5,
6]. Furthermore, life cycle analysis has also been carried out for different hydrogen production methods [
7,
8]. In both these studies, the environmental impacts of hydrogen production through electrolysis are compared with different energy sources, and both find electrolysis powered by renewable sources such as wind and solar are the most beneficial [
7,
8]. Recently, there has also been growing interest in using algae to produce hydrogen [
9].
A large number of studies and reviews have investigated how to improve hydrogen production through electrolysis of water. The review of Wang et al. looks in detail at different amorphous materials that can be used as electro-catalysts for water splitting [
10]. Olivier et al. review and discuss the modelling and model equations required, including different analytical and empirical relations used [
11]. Hu et al. also reviews modelling approaches focusing on alkaline water electrolysis [
12]. In addition to giving model equations, they also discuss the importance of different operating conditions such as temperature, pressure, electrolyte flow, and concentration [
12]. They also list a range of different ways by which water electrolysis can be enhanced, including modifying operating conditions, changing the design/geometry of the electrolyzer, using different catalysts, and applying external fields (gravity, magnetic, ultrasound, and pulsing electric fields) [
12].
The review of Li et al. goes into great depth discussing the theory and mechanisms by which magnetic fields can enhance water electrolysis [
13]. They mention the following three main effects [
13]:
Magneto-hydro-dynamic (due to the Lorentz force)
Magneto-thermal effect (in alternating magnetic fields)
Interactions with spin states of molecules (in the electrode reactions)
Most recently, Sun et al. have also discussed these effects and some of the latest results that have been obtained from applying magnetic fields to water electrolysis [
14]. Both of these reviews provide detailed discussion of the theory and provided examples from the literature where magnetic fields have provided some enhancement [
13,
14]. However, they have offered only limited comparisons of the different enhancements obtained by these methods [
13,
14]. In most cases, the enhancements are discussed separately for each existing study and attributed to one of the three main effects mentioned above. In addition, both Sun et al. and Li et al. point out that there may be multiple effects in play when applying a magnetic field, so it is difficult to assess the combined effects, requiring further study [
13,
14].
This lack of a complete and unified theory for explaining the combined effects under magnetic fields makes it difficult to predict the resulting overall enhancement. Rather than relying on theory, this review looks at the empirical results and findings of different studies which investigated the influence of magnetic fields on water electrolysis. Here, the aim is to compare the different enhancement effects obtained with different types and configurations of magnetic fields. In particular, the two types of magnetic field considered are static (typically uniform) and alternating magnetic fields. For each type, the enhancement effects are compared such that limitations can be identified, and recommendations can be made about future directions of research.
Section 2 gives basic details about the design and modelling of electrolysis systems, highlighting inefficiencies/limitations.
Section 3 examines the empirical performance of enhancements through the application of external magnetic fields, and
Section 4 discusses the limitations of existing work in this field and gives recommendations for future research in the area of magnetic field-assisted water electrolysis.
2. Water Electrolysis Systems Design and Limitations
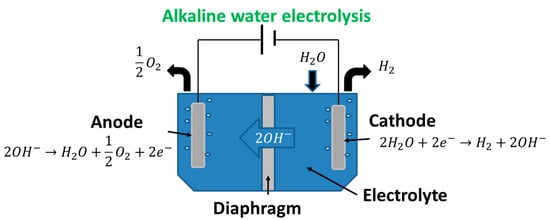
Water electrolysis systems are all based on the water splitting reaction as shown in Equation (1). This overall reaction relies on electrons being transferred through an electric circuit from the anode to the cathode. However, the reactions which occur at the anode and cathode depend on the type of electrolysis system, with one of the common designs and the associated reactions shown in
Figure 1. This type of electrolysis is defined as one of the “mature” methods in terms of technological readiness [
15]. In this review, the focus is on enhancing the most mature existing technology, which is alkaline electrolysis.
The energy for water splitting (shown in Equation (1)) is provided by the voltage applied. The Gibbs free energy
of this overall reaction is related to the reversible voltage
as defined by Equation (2) [
16]:
where
z is the number of electrons transferred and
F is the Faraday constant [
16]. The Gibbs free energy depends on the operating temperature and pressure [
17]. Correlations are available for predicting this where it can be seen that
reduces with increasing temperature [
12]. However, the actual voltage used for the electrolysis cell
must be higher than this for a number of reasons. Firstly, there will be an irreversible change in entropy
such that voltage is increased to
where is the change in enthalpy related to Gibbs free energy through and is the thermoneutral cell voltage [16]. In addition to this, the voltage must be increased further to account for various resistances in the system. These resistances translate to increases in the voltage called overpotentials. Figure 2 shows a typical voltage–current density diagram which shows how voltage changes increase current density.
The overpotential can be broken down into ohmic overpotentials (related to factors such as electrolyte concentration, electrode gap, bubble coverage on electrodes, temperature, and pressure) and activation overpotentials (related to factors such as electrode materials, bubble coverage, and current density) as well as diffusion overpotentials [
12]. Combining all these terms, the overall cell voltage can be calculated by Equation (4) [
12]:
where and are the activation overpotentials of the cathode and anode, which may have different electro catalyst materials, is the ohmic overpotential, and is the diffusion overpotential. While detailed analytical models are available for estimating the separate parts of the overpotential, there are also empirical models for estimating the overall overpotential [11].
The hydrogen production rate
can be obtained using Faraday’s law as shown in Equation (5) [
11]. This says that the hydrogen production is proportional to the current flowing through the system
.
However, due to current losses in the system, sometimes called parasitic losses, it may not be possible to convert 100% of the current supplied into hydrogen production [
10]. To account for this difference, the Faraday efficiency
can be inserted into Equation (5) to give Equation (6) [
16]. Hence, Equation (6) can be used to calculate the hydrogen production from a single electrolysis cell (multiplying by the number of cells to give the total hydrogen production [
16]. For alkaline electrolysers the Faraday efficiency can be calculated through empirical correlations [
11].
The total power consumption of the electrolyser can be calculated by multiplying the cell voltage and the current as shown in Equation (7). Looking at the above discussion and equations, it can be seen that there are two main sources of inefficiency in these systems:
Both of these affect the overall energy efficiency of hydrogen production through electrolysis. Typically, when attempting to improve efficiency, research to reduce overpotentials focuses either on overpotentials associated with the hydrogen evolution reaction (HER) at the cathode or the oxygen evolution reaction (OER) at the anode [
18].