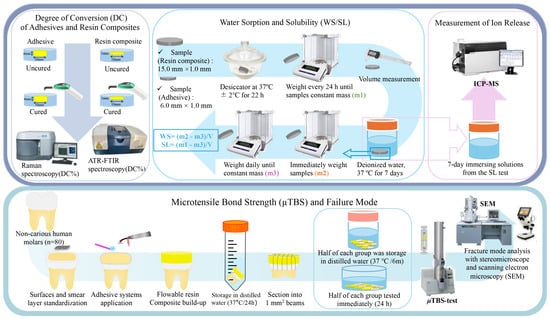
1. Introduction
Universal adhesives (UAs) are widely recognized as adhesive systems that can be applied in etch-rinse and self-etch modes to various dental tissues and materials utilized in direct or indirect restorative techniques [1]. Due to their multifunctional features, their composition is more complex than the preceding all-in-one self-etch adhesive systems. When developing single-bottle UAs, an ideal mixture of acrylic resin monomers with differing hydrophilicity and hydrophobicity, solvent water, silane, and functional monomers needed to be blended and function well in combination, ideally polymerized to produce a durable bonded interface [2]. However, achieving an ideal composition is highly technique-sensitive since all adhesive compounds blend, even though they are not always miscible [3].
A functional monomer, 2-hydroxyethyl methacrylate (HEMA), is frequently added to UAs. HEMA is highly soluble in other solvents and water; its hydrophilic and co-solvent nature improves stability and helps to keep hydrophobic and hydrophilic monomers in a homogenous solution by reducing phase separation [4]. Phase separation occurs when the solvent evaporates at moist bonding interfaces, especially when using adhesives like UAs that contain high water concentrations [1]. Moreover, HEMA’s polar characteristics and water-solubility properties increase wettability and promote resin monomer diffusion into the dentin collagen fibril network [5]. However, uncured HEMA also lowers water vapor pressure in the adhesive and may make it more challenging to evaporate during the air-drying step, impairing polymerization [6]. Due to its high hydrophilicity, HEMA readily absorbs water in its uncured and polymerized state [7]. High water sorption can cause a decrease in mechanical properties and contribute to the degradation of dental polymer matrices [5]. A previous investigation has also suggested that HEMA hampers the interactions between the phosphate groups of 10-MDP and hydroxyapatite (HAp), which could undermine the bond strength of UAs containing substantial amounts of HEMA [8]. To increase the durability of the composite restoration, manufacturers and researchers have attempted to reduce the hydrophilicity of UAs by adjusting the concentrations of HEMA to increase the longevity of the composite restoration [9]. UAs could still be considered a one-step adhesive [2].
The so-called controversial bioactivity has been another property addressed in dental restorative materials for a stable dentin-resin bonding [1]. The latest definition of bioactivity from FDI divides the mechanisms into three levels: purely biological, mixed biological/chemical, or strictly chemical (e.g., through ion release from bioactive glass fillers [10]). Recently, significant interest has been drawn to using glass particles that demonstrate various effects by releasing multiple ions [11]; among them, the silanated pre-reacted glass ionomer (S-PRG) filler has been widely used in commercial products. According to the manufacturer, the S-PRG filler is a multilayered, ultrafine glass particle with a SiO2 coating on the outer layer, a pre-reacted glass-ionomer phase in the middle, and a glass core that could be released into dental hard tissues and enhance their mineralization [12].
The cured adhesive layers in single-step adhesives may act as semi-permeable membranes that allow water diffusion from the bonded hydrated dentin to the intermixed zone between the adhesive and the composite [13]. A recent study reported that multi-ions released by S-PRG-filled resin composite might permeate tooth substrates through adhesives, hindering tooth demineralization around composite restorations [14]. To combine the advantages of UAs and the properties of S-PRG fillers, new resin-based products have been developed and require investigation. Therefore, this study aimed to investigate the effects of a resin composite (RC) containing S-PRG filler on the dentin bond strength of HEMA-free and HEMA-containing UAs over a period of 6 months. Two different two-step self-etch adhesives (2-SEAs) were used as reference groups. Additionally, the water sorption and solubility of the materials, as well as their degree of conversion, were investigated. The hypotheses were that (1) there would be no significant differences in the degree of conversion of materials; (2) there would be no difference in the water sorption and solubility of adhesive systems and RCs; (3) there would be no difference in the ion release of adhesives and RCs; (4) the storage period, adhesive types, RCs, and their combinations would not affect the dentin bond strength to dentin.
4. Discussion
The DC of resin-based materials is influenced by filler ratio, photo-initiators, monomer and co-initiator properties, and light-curing conditions [19,20]. This study supports evidence from previous observations that there was no significant difference in the DC between BFP and other resin composites [21,22]. Thus, the first hypothesis can be rejected based on the DC results. The restorative resin composites in this study were similar in composition, as can be seen in their spectra (Figure 2), except for their inorganic content. Hence, it may be possible to infer that the filler type did not affect the DC of the tested resins. Regarding the adhesives, E-BBX2’s DC was significantly lower than that of BBX, and these results are consistent with previous reports [23]. According to this result, it could be suggested that, as HEMA concentration increased, DC declined [23], as observed in Figure 2 and Table 2. This may be due to the lower polymer reactivity of monomethylated HEMA [5]. Also, the hydrophobic photo-initiators and co-initiators in the UAs might not be compatible with hydrophilic HEMA, resulting in a lower DC [24]. HEMA-free UAs demonstrated a DC compatibility with the 2-SEAs [25], corroborating our findings.
The diffusion process regulates the water sorption and solubility of restorative materials. The absorption of water by the polymer matrix and leaching can lead to the separation of the filler from the matrix or even the deterioration of the fillers through hydrolysis [5]. Typically, water absorption is reduced in materials with a higher filling level but is also affected by the composition of monomers [26]. ISO 4049:2019 recommends that the WS of resin-based materials be ≤40.0 μg/mm3, with which both restorative resin composites (i.e., BFP and E-BFP) comply. BFP’s WS agrees with previous findings and is higher than E-BFP, which could be attributed mainly to the S-PRG fillers [22]. The pre-reacted glass-polyacid zones inside the BFP S-PRG filler’s structure may create an osmotic action that causes swelling and pressure [27], which could explain BFP’s WS result. The WS of all adhesives failed to comply with the ISO 4049:2019 threshold. As only the bonding resins of FBII and E-FBII were measured, they resulted in a lower WS than the other UAs. These materials mainly comprise UDMA, which is more hydrophobic than Bis-GMA. This is because Bis-GMA contains more hydrophilic hydroxyl groups of Bis-GMA, which form stronger hydrogen bonds with the water molecules than the urethane groups of UDMA, thus resulting in low WS values [28,29]. The WS of the UAs is influenced by the hydrophilic and acidic functional monomers as well as the composition and concentration of the solvent [5]. One potential reason for the intriguing outcome of HEMA-free BBX displaying a similar WS to E-BBX1 and E-BBX2 is its solvent, acetone. Although BBX is HEMA-free, the high contents of acetone, water, and functional monomer result in high hydrophilicity, like that of the other two HEMA-containing UAs. Similar findings were reported concerning another HEMA-free universal, which showed higher WS than other UAs [25]. The high WS of UAs could be attributed to water bonded to polar regions of the polymer through hydrogen bonds [30]. Regarding the SL, only BFP, E-BFP, FBII, and E-FBII, composed of hydrophobic monomers, complied with the ISO 4049:2019 recommendation (≤7.5 μg/mm3). The S-PRG fillers in BFP are more soluble and easily released than those silica fillers contained in E-BFP, thus explaining the higher SL than E-BFP [31]. The SL of BBX, E-BBX1, and E-BBX2 greatly exceeded FBII and E-FBII, coherent with the high content of solvents, hydrophilic and/or ionic monomers in universal adhesives [32,33]. Thus, considering the WS and SL findings, the second null hypothesis was rejected.
Ion release from dental materials is beneficial to stabilizing the collagen matrix, stimulating remineralization, and reinforcing dentin, as depicted in a recent study [34]. In our study, the ion release measurement demonstrated that BFP resin composite exhibited a higher release of B, Si, Sr, and F ions than E-BFP resin, which was corroborated by Shimizu et al. [17]. Nevertheless, in previous investigations, dentin pretreated with S-PRG filler eluent showed greater tensile strength than that treated with NaF [34]. The F, Na, and Sr ions can enhance enamel and dentin’s mechanical strength and acid resistance by forming fluorinated and sodium/strontiated hydroxyapatite [11]. Furthermore, it has been suggested that Sr and F ions could facilitate remineralization and suppress matrix metalloproteinase activity [12]. On the other hand, incorporating boron in the hydroxyapatite structure (mainly as borate substituting phosphate and OH groups) positively impacts dentin by providing antimicrobial protection and promoting remineralization, thereby restoring dentin’s mineral content and structural integrity [11]. A previous study demonstrated that combining the universal adhesive BeautiBond Xtrene with a resin composite containing 70 wt% S-PRG filler increased the hardness of the dentin around the restoration, probably due to the release of B and F ions. [14]. Additionally, FBII, the only adhesive containing S-PRG filler, exhibited a greater B and F ions release than the others. It could be suggested that due to the absence of silica fillers, the three UAs showed lower Si ion release than the other 2-SEA that incorporate fillers. The ion release pattern seen in this study aligns with findings from earlier investigations [11]. The non-detectable release of Al ion was also previously reported [35]. It is relevant to highlight that failure to detect ions released from S-PRG fillers-containing materials does not necessarily mean there is no release, as detection limits immersion liquids, and ratios could impact the ability to detect the actual ions’ leaching [14]. Similar results to the current study were obtained in recent research, where an injectable resin composite containing S-PRG fillers released significantly more B, Sr, and F ions than the silica-filled material, and it was comparable to BFP, possibly [36]. Imazato et al. have demonstrated that materials with S-PRG fillers, such as RC, adhesive, and resin cement, can continue to release ions for more than 1 year, even though the release patterns may vary [11].
The µTBS results indicated that restorative resin composite and storage period significantly influenced the bond strength of the adhesives to dentin; hence, the third null hypothesis was rejected. Regarding the 24 h µTBS, E-BBX1 and E-BBX2 presented higher values when utilizing BFP. This finding accords with earlier observations, which showed that differences in the mechanical properties caused by various compositions of restorative resin composites could influence the µTBS to dentin [37,38]. The critical difference between BFP and E-BFP resin composites is the filler type, as BFP contains S-PRG filler, and E-BFP is loaded with silica. It has been reported that the dentin shear bond strength of Beautifil II, which also contains S-PRG filler, is significantly greater than that of other bioactive restorative materials [39]. This different behavior is attributed to the high concentration of S-PRG filler (83.3 wt%) and the resin’s minimal amount of volumetric shrinkage [39]. In addition, the positive effect of BFP’s S-PRG filler content on E-BBX1 and E-BBX2 might be related to the HEMA content in these universal adhesives, which makes them behave as semi-permeable membranes [1]. Indeed, ions released from S-PRG-filled resins could penetrate UA’s semi-permeable membranes and reach tooth substrates at the cavity wall [40]. This ion-releasing process might prevent microbial leakage into the adhesive interface, contributing to the inhibition of demineralization around resin composite restorations [14,40] and creating more durable bonds [41,42]. Nevertheless, the restorative resin composite did not affect the bond strength of the BBX, which may be attributed to the fact that the absence of HEMA may not have resulted in a further thinning of the interfacial thickness compared to the other two UAs to which HEMA was added [43]. Moreover, strong air-blowing during BBX application might have removed the water at the interface, resulting from the adhesives’ components’ phase-separation and generating few defects in the bonded layer [44].
Conversely, the impact of the restorative resin seems less significant on the bonding capabilities of 2-SEAs compared to UAs. As 2-SEAs, FBII and E-FBII present a separate resin bonding layer, and their overall thickness is higher than that of UAs, which is usually less than 10 µm [1], favoring the absorption of interfacial tension and stress. Most adhesive failures in the FBII and E-FBII showed complete debonding of the adhesive layer from dentin (Figure 4 and Figure 5), which implies that thicker adhesive layers may be more robust and fracture-resistant.
Interestingly, the bond strength of all adhesives to dentin was similar at 24 h when the same resin composite was utilized. The tested UAs presented HEMA concentrations from 0 to 10%, and the role of concentration variation is not precisely correlated with the bonding behavior of adhesives as other components also vary among the adhesives (e.g., solvents, functional monomers, filler content) [30,45]. Low concentrations of HEMA (10%) have been described to increase the 24 h dentin µTBS in one-step SEA, supposedly due to improved wetting properties [46]. Contrarily, UAs’ stable dentin bonds have been reported over time with 2.5–10% HEMA content [45], which follows our findings.
After 6 m of water storage, the µTBS levels of the E-BBX1 and E-BBX2 used with BFP were significantly lower than the 24 h µTBS. Furthermore, there was no difference between BFP and E-BFP after 6 m storage, irrespective of the adhesives. The possible explanation for the 6 m bond strength decrease of E-BBX1 and E-BBX2 with BFP also relies on the higher WS of the BFP compared to the E-BFP and the long-term detrimental effects of HEMA hydrophilicity. Recent research has indicated that resins with S-PRG fillers experience a notable decrease in flexural strength after 30 days of water storage compared to 1 day, likely due to the greater WS [36,47]. A similar result was observed in another investigation when the bond strength of Beautifil II decreased as WS increased during prolonged water storage [48]. Tang et al. (2024) have reported that the presence of HEMA in the UAs led to an increase in WS and a decrease in µTBS after aging. However, the HEMA-free UAs showed no significant changes in WS and µTBS after 50,000 thermocycles, regardless of application mode [30]. Furthermore, a significant reduction in the µTBS of HEMA-containing UAs following thermal cycling was related to the enhanced water permeability resulting from the lower levels of DC and HEMA present [49], consistent with the E-BBX2 results in our study.
The outcomes of this study should be carefully considered, as limitations in its design need to be mentioned. Chemical analyses were not performed on the dentin to determine if passage and deposition of released ions from the composite resin occurred. In addition, to ensure minimal possible variation in components, all tested materials were obtained from the same manufacturer. Hence, subsequent research should add materials from other manufacturers to assess compatibility between different products
Source link
Di Wu www.mdpi.com