1. Introduction
This study introduces an innovative approach by developing a MnO2@CeO2 nanozyme that mimics laccase activity, enhancing the electrochemical detection of phenolic compounds. The integration of MnO2 into CeO2 nanorods significantly boosts their catalytic performance, increasing laccase-like activity by 300%. Leveraging this high activity, a novel electrochemical sensor was created for the rapid and sensitive detection of hazardous phenolic compounds like bisphenol A and catechol in red wine. By modifying a glassy carbon electrode with polyethyleneimine, the sensor achieves ultra-low detection limits, offering a highly sensitive, cost-effective, and stable method for food safety analysis. This method represents a significant advancement in the use of nanozymes for foodborne contaminant detection.
2. Materials and Methods
2.1. Reagents, Characterization Techniques
Ethanol, Guaiacol, 2,4-Dichlorophenol (2,4-DP) and 4-Aminoantipyrene (4-APP) were bought from Shanghai Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. BPA, CC, KMnO4, CeCl3·7H2O, and polyethyleneimine (PEI) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Exhibiting a Km value of 0.4 mM and a Vmax value of 3 µM, laccase was obtained from Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China. In this study, all chemicals were analytically pure, and the laccase used was a purified enzyme solution derived from Trametes versicolor. The methanol and formic acid used in the chromatographic analysis were of HPLC grade, purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China.
Electrochemical curves were measured by an electrochemical workstation (Chen Hua Instruments Co., Shanghai, China), using different signal transducers of cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Transmission electron microscopy (TEM) images were measured by a TEM (Hitachi High-Technologies Co., Ltd., Tokyo, Japan). X-ray diffraction (XRD, Shimadzu Enterprise Management China Co., Ltd., Tokyo, Japan) patterns were recorded on the X-ray powder diffractometer. An energy dispersive spectrometer (EDS, Shanghai Jingke Scientific Instrument Co., Ltd., Shanghai, China) was used to characterize the MnO2@CeO2 nanozyme together with TEM. Absorption spectra were performed on a UV-vis spectrophotometer (UV-1800, AoYi Instruments Shanghai Co., Ltd., Shanghai, China). An enzyme-labeled instrument was provided by Gene Co., Ltd., Hongkong, China.
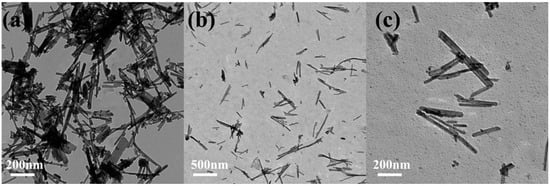
2.2. Synthesis of MnO2@CeO2 Nanozyme
In a typical synthesis, for 8% Mn4+-doped CeO2 NRs, 0.4 g CeCl3·7H2O and 0.012 g KMnO4 was dissolved in 30 mL of 9 mol/L NaOH solution under vigorous stirring. The suspension was transferred to a 50 mL Teflon-lined stainless-steel autoclave and held at 140 °C for 48 h. After the autoclave was cooled to room temperature naturally, fresh precipitates were separated by centrifugation and washed with deionized water to neutrality and with ethanol several times. The MnO2@CeO2 nanozymes were obtained by drying the precipitates at 60 °C overnight.
2.3. Evaluation of the Catalytic Performance of Nanozyme and Laccase
The catalytic activity of the MnO2@CeO2 nanozyme and laccase was determined using the colorimetric reaction between 2,4-DP and 4-APP; 2,4-DP (0.1 M, 10 µL) and 4-APP (0.1 M, 10 µL) was mixed with MnO2@CeO2 nanozyme aqueous dispersion (1 mg/mL, 80 µL) or laccase (10 mg/mL, 60 µL). Then, Tris-HCl buffer (0.1 M, pH 7.0, 180 µL) was added in the mixture up to 200 µL. The reaction was maintained at 37 °C for 2 h, and then, the absorbance was detected at 485 nm.
2.4. Determination of the Catalytic Kinetic Parameters
The prepared MnO2@CeO2 nanozyme (1 mg/mL, 80 μL) or laccase (10 mg/mL, 60 μL) was mixed with 2,4-DP (0.1 M, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 µL) and 4-APP (0.1 M, 15 µL). Tris-HCl buffer (0.1 M, pH 7.0, 180 µL) was added in the mixture up to 200 µL. At 37 °C, the kinetics of the reaction can be determined by monitoring the change in the absorption wavelength at 485 nm over time by ultraviolet-visible spectroscopy. All the experiments were repeated thrice.
2.5. Evaluation of the Stability of Catalyst
The MnO2@CeO2 nanozyme or laccase was incubated at varying pH (3.0–9.0) for 7 h to evaluate the effect of pH on catalytic activity. In order to study the temperature stability of the MnO2@CeO2 nanozyme and laccase, they were stored at −18~120 °C for 45 min before determining their catalytic activity. The catalytic activity at 30 °C was used as a reference. In the same way, the effect of organic solvents on catalytic activity was evaluated by the addition of different amounts of methanol (0, 10%, 20%, 40%, 60%, 80%, and 100% v/v) in the reactants. The absorbance of the supernatant at 485 nm was measured after 2 h.
2.6. Preparation of MnO2@CeO2/GCE
A total of 5 mg of MnO2@CeO2 nanozyme and 25 mg of PEI were added into 5 mL double distilled water and sonicated for 30 min. The GCEs were polished with 0.05 µm and 0.3 µm alumina pastes, then washed with distilled water and dried at 26 °C. A total of 5 µL of 1 mg/mL MnO2@CeO2 nanozyme suspension was dropped on the GCEs and then dried at room temperature. The dried modified electrode was used for the electrochemical detection of BPA and CC.
2.7. Electrochemical Experiments
A Tris buffer solution of 0.1 mol L−1, pH 7.0 was used as the electrolyte solution at room temperature. A three-electrode system was formed with the MnO2@CeO2/GCE electrode, with the GCE electrode as the working electrode, Pt wire as the main auxiliary electrode, and Ag/AgCl as the reference electrode. The size of the electrolytic cell was 5 mL, which was purchased from Guangzhou Saios Chemical Instrument Co., Ltd., Guangzhou, China. DPV was used with a pulse amplitude of 50 mV, a pulse width of 0.05 s, a potential increment of 4 mV, a pulse cycle time of 0.5 s, a sensitivity of 1e−5 A V−1, and a scanning in the negative direction at a potential in the range of 0.2–1.4 V with a scanning speed of 100 mV s−1, and the DPV curve was recorded.
2.8. High-Performance Liquid Chromatography (HPLC) Analyses
The HPLC analysis of BPA was conducted using a HPLC instrument (Thermo Fisher UltiMate 3000, Waltham, MA, USA) equipped with a reversed-phased column Syncronis C18 column (100 mm × 2.1 mm, 1.7 μm) maintained at a constant temperature of 30 °C with a diode array detector (DAD) set at 280 nm. The analysis involved the injection of 10 μL of 0.22 μm membrane-filtered samples at a flow rate of 0.2 mL/min, and the solvents consisted of a methanol–water mixture in a ratio of 65:35 (v/v).
A HPLC system (Prominence LC-20A, Shimadzu, Japan) with an analytical column Venusil MP C18 (4.6 mm × 250 mm, 5 μm) was used at a constant temperature of 35 °C. The isocratic elution was performed using a 5 mM ammonium acetate−1‰ formic acid solution (solvent A) and methanol (solvent B) in a ratio of 30:70 (v/v). The injection volume was 10 μL of 0.22 μm membrane-filtered samples, with a flow rate of 1 mL/min.
Source link
Chao Wang www.mdpi.com